Radioligands targeting the prostate-specific membrane antigen (PSMA) are currently used in the clinics to treat patients with metastatic castration-resistant prostate cancer. Continuous investigations are, nevertheless, conducted to design new small molecule-based radioligands and improve their respective pharmacokinetic properties. Various strategies have been devised to reasonably prolong the blood circulation, which would result into enhanced tumor accumulation and radiation dose delivered to eliminate the cancer cells. The goal of this study was to investigate the influence of the incorporation of a transthyretin binder (TB-01) in the tumor uptake of the resultant PSMA-targeted radioligand.
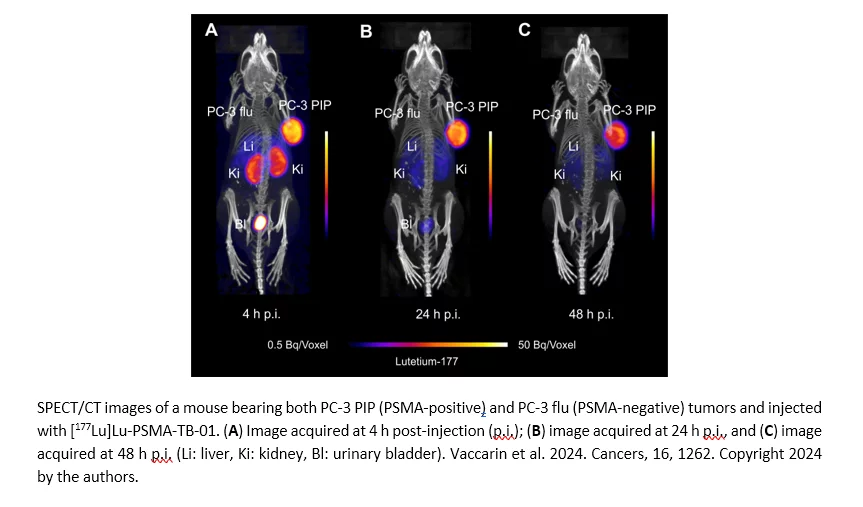
Transthyretin binders have previously been used to improve the pharmacokinetic properties of small-molecule drug conjugates and could, thus, be utilized for radiopharmaceuticals as an alternative to the widely explored “albumin binder concept”. In this study, a novel PSMA ligand modified with a transthyretin-binding entity (TB-01) was synthesized and labeled with lutetium-177 to obtain [177Lu]Lu-PSMA-TB-01. A high and specific uptake of [177Lu]Lu-PSMA-TB-01 was found in PSMA-positive PC-3 PIP cells (69 ± 3% after 4 h incubation), while uptake in PSMA-negative PC-3 flu cells was negligible (<1%). In vitro binding studies showed a 174-fold stronger affinity of [177Lu]Lu-PSMA-TB-01 to transthyretin than to human serum albumin. Biodistribution studies in PC-3 PIP/flu tumor-bearing mice confirmed the enhanced blood retention of [177Lu]Lu-PSMA-TB-01 (16 ± 1% IA/g at 1 h p.i.), which translated to a high tumor uptake (69 ± 13% IA/g at 4 h p.i.) with only slow wash-out over time (31 ± 8% IA/g at 96 h p.i.), while accumulation in the PC-3 flu tumor and non-targeted normal tissue was reasonably low. Further optimization of the radioligand design would be necessary to fine-tune the biodistribution and enable its use for therapeutic purposes. This study was the first of this kind and could motivate the use of the “transthyretin binder concept” for the development of future radiopharmaceuticals.
Contact
Dr. A.K. Mapanao
Paul Scherrer Institut
Forschungsstrasse 11
5232 Villigen PSI
+41 56 310 56 03
ana.mapanao@psi.ch