Surface oxidation/spin state determines oxygen evolution reaction activity of cobalt-based catalysts in acidic environment
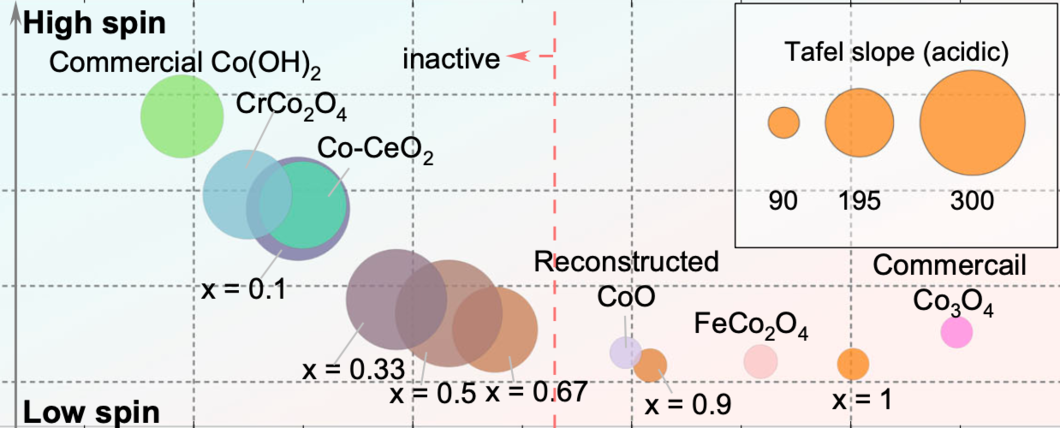
Co-based catalysts are promising candidates to replace Ir/Ru-based oxides for oxygen evolution reaction (OER) catalysis in an acidic environment. However, both the reaction mechanism and the active species under acidic conditions remain unclear. In this study, by combining surface-sensitive soft X-ray absorption spectroscopy characterization with electrochemical analysis, we discover that the acidic OER activity of Co-based catalysts are determined by their surface oxidation/spin state.
Surfaces composed of only high-spin CoII are found to be not active due to their unfavorable water dissociation to form CoIII- OH species. By contrast, the presence of low-spin CoIII is essential, as it promotes surface reconstruction of Co oxides and, hence, OER catalysis. The correlation between OER activity and Co oxidation/spin state signifies a breakthrough in defining the structure-activity relationship of Co-based cata- lysts for acidic OER, though, interestingly, such a relationship does not hold in alkaline and neutral environments. These findings not only help to design efficient acidic OER catalysts, but also deepen the understanding of the reaction mechanism.