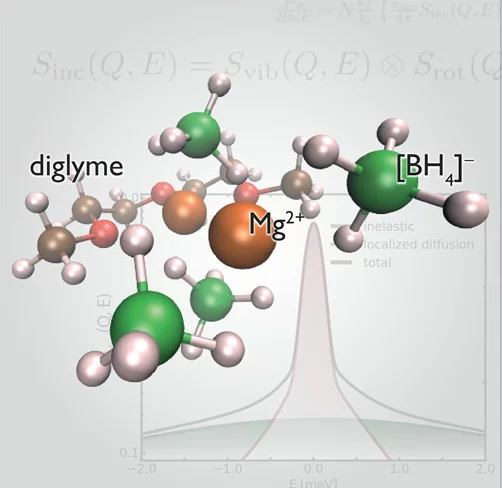
Coordination complexes of magnesium borohydride show promising properties as solid electrolytes for magnesium ion batteries and warrant a thorough microscopic description of factors governing their mobility properties. Here, the dynamics of Mg(BH4)2-diglyme0.5 on the atomic level are investigated by means of quasielastic neutron scattering supported by density functional theory calculations and IR and NMR spectroscopy. Employing deuterium labeling, we can unambiguously separate all the hydrogen-containing electrolyte components, which facilitate Mg2+ transport, and provide a detailed analytical description of their motions on the picosecond time scale. The planar diglyme chain coordinating the central Mg atom appears to be flexible, while two dynamically different groups of [BH4]− anions undergo reorientations. The latter has important implications for the thermal stability and conductivity of Mg(BH4)2-diglyme0.5 and demonstrates that the presence of excess Mg(BH4)2 units in partially chelated Mg complexes may improve the overall performance of related solid-state electrolytes.
Reference: T. Burankova et al, Journal of Physical Chemistry Letters 9, 6450 (2018)
Read full article: here