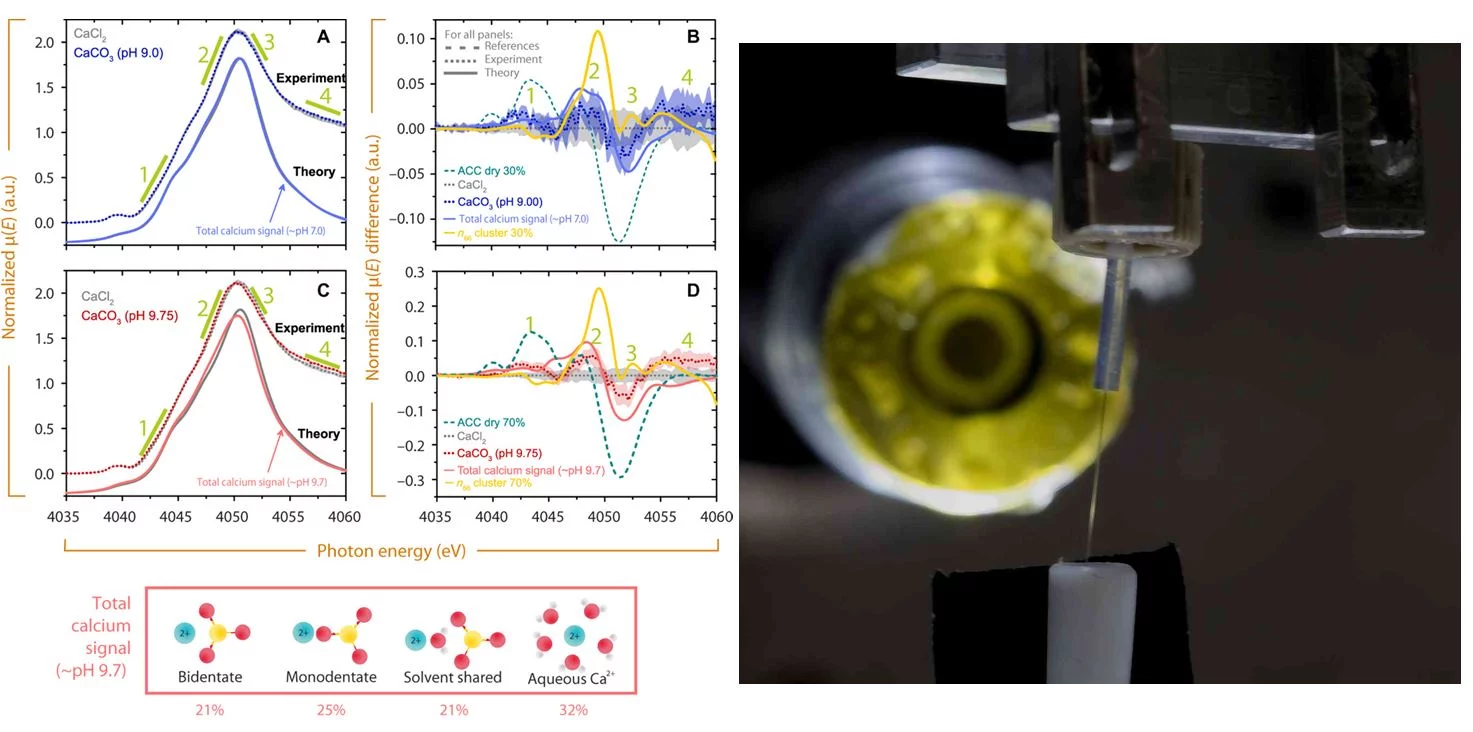
Despite its widespread occurrence in nature and its importance in a variety of industrial applications, the nucleation and subsequent crystallization of calcium carbonate is still a matter of debate. Classical theory predicts that supersaturated carbonate solutions consist mostly of ions and ion pairs, with a small number of larger clusters present in the solution. The population of the different sized clusters in a solution is solely defined by the cluster’s size dependent Free Energy. If clusters are large enough they serve as nucleation germs for a new solid phase. The nucleation occurs once the surface free energy barrier posed by the new solid-liquid interface is overcome by the free energy win from bulk phase growth. However, for calcium carbonate solutions, this classical view has been challenged by the emergence of non-classical theories. The most predominant of these non-classical theories postulate that calcium carbonate solutions contain oligomeric species in amounts exceeding predictions from classical solution models and the agglomeration of these oligomers result in crystal nucleation and growth. In a novel experiment, using synchroton X-ray absorption spectroscopy, the molecular structure around the solvated calcium ion in dilute supersaturated calcium carbonate solutions was probed in situ. The results obtained definitively show that supersaturated calcium carbonate solutions are dominated by ions and ion pairs. The presence of larger oligomers as predicted by non-classical theories was not detected. Moreover, predictions from independent theoretical calculations on the structure of supersaturated calcium carbonate solutions were in congruence with the experimental results. As a consequence, it is quite clear that the nucleation of calcium carbonate follows a classical pathway. Apart from offering new insights into a fundamental and ubiquitous process, this research also unveils state of the art techniques for both experimental and theoretical calculations. The diversity of the team of scientists with different viewpoints and experiences played a key role in addressing this important research question. The team consisted of theoreticians from DOE’s Pacific Northwest National Laboratory, University of Minnesota and experimentalists from Paul Scherrer Institut in Switzerland.
Contact
Dr. Thomas HuthwelkerSwiss Light Source
Paul Scherrer Institut
Telephone: +41 56 310 5314
E-mail: thomas.huthwelker@psi.ch
Dr. Christopher J. Mundy
Physical Sciences Division, Pacific Northwest National Laboratory, Richland, WA 99354, USA and Department of Chemical Engineering, University of Washington, Seattle, WA 98195, USA.
E-mail: chris.mundy@pnnl.gov
Dr. James J. De Yoreo
Physical Sciences Division, Pacific Northwest National Laboratory, Richland, WA 99354, USA and Department of Materials Science and Engineering, University of Washington, Seattle, WA 98195, USA.
E-mail: james.deyoreo@pnnl.gov
Original Publication
Supersaturated calcium carbonate solutions are classicalKatja Henzler, Evgenii O. Fetisov, Mirza Galib, Marcel D. Baer,Benjamin A. Legg, Camelia Borca, Jacinta M. Xto, Sonia Pin, John L. Fulton, Gregory K. Schenter, Niranjan Govind, J. Ilja Siepmann, Christopher J. Mundy, Thomas Huthwelker, James J. De Yoreo
Science Advances, 26 Jan 2018
DOI: 10.1126/sciadv.aao6283