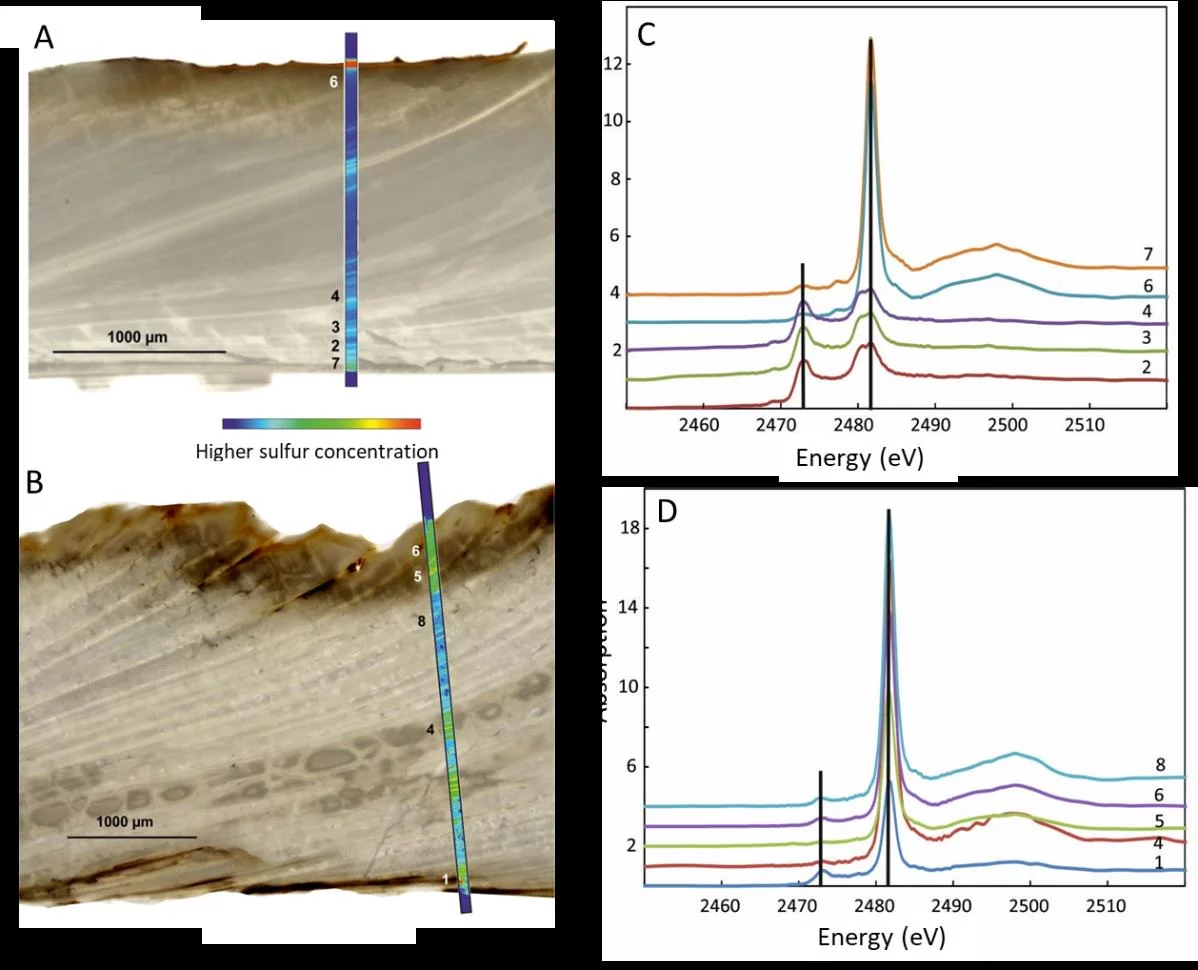
Marine carbonates (biogenic shells and bulk rocks) are the most important archives for geochemical signatures of former oceans. Although isotopic, elemental, and mineralogical signals are widely used to reconstruct the past seawater properties the effects of post-depositional processes on these signals are not fully understood. Sulfate that is incorporated in marine carbonates is an important tool for reconstructing the marine sulfur cycle. Still, uncertainties persist where exactly in the carbonate the so-called carbonate associated sulfate (CAS) is located and how sulfate in carbonate rocks reacts on alteration processes. Possible effects could include sulfate loss or exchange with dissolved sulfate in the pore water, oxidation or reduction of different sulfur phases, and a change in the isotopic ratio of different sulfur phases occurring in the carbonate. To simulate the chemistry of the alteration, shells of the marine bivalve Arctica islandica were artificially altered in modified seawater at temperatures of 100°C and 175° for a durations between one and twelve weeks. The multi-analytical approach of XANES and µ-XRF analyses, sulfur isotope measurements, NanoSIMS analyses, and microstructural analysis on thin-sections of the shell samples revealed how sulfur in a bivalve shell reacts to artificially induced hydrothermal alteration. For this endeavor, the chemical sensitivity of XAS, which allows to determine the chemical state of sulfur with micrometer spatial precision played a key role. High-resolution sulfur µ-XRF maps revealed the sulfur distribution in a cross section of a shell from the inner to the outer shell layer and along annual growth bands. Sulfur XANES analyses showed that CAS in A. islandica is indeed incorporated into the mineral part of the pristine shell, most likely as a hydrated or partly hydrated sulfate phase. Reduced sulfur phases are found only in the intra-shell organic matrix. Hydrothermal alteration at 175°C oxidizes these reduced phases, and destroys the intra-shell organic matter (see figure). As a further consequence of hydrothermal alteration total sulfur concentrations in the shells decreased and the distribution pattern changed as displayed by µ-XRF maps (see figure). Together with microstructural analyses, the results of the present study indicate that sulfur distribution and lower concentration reflect the formation of new carbonate phases with a sulfur absence in the newly formed calcite crystals. The study shows that sulfur is present in the intrashell organic matter in mainly reduced states and in the mineral (carbonate) shell part as sulfate. This chemical variety is the main reason that sulfur is a sensitive and useful proxy for diagenetic alteration in biogenic carbonates.
Contact
Dr. Vanessa FichtnerDept. for Earth and Environmental Sciences
University of Kentucky
121 Washington Avenue
Lexington, Kentucky 40506 USA
E-mail: vanessa.fichtner@uky.edu
With financial support from project CHARON - Phase II
Prof. Dr. Harald Strauß
Institut für Geologie und Paläontologie - Historische und Regionale Geologie
Westfälische Wilhelms-Universität Münster
Corrensstr. 24
D-48149 Münster
hstrauss@uni-muenster.de
Dr. Thomas Huthwelker
Swiss Light Source
Paul Scherrer Institut
Telephone: +41 56 310 5314
E-mail: thomas.huthwelker@psi.ch
Original Publication
Incorporation and subsequent diagenetic alteration of sulfur in Arctica islandicaVanessa Fichtner, Harald Strauss, Vasileios Mavromatis, Martin Dietzel, Thomas Huthwelker, Camelia N. Borca, Paul Guagliardo, Matt R. Kilburn, Jörg Göttlicher, Chelsea L. Pederson, Erika Griesshaber, Wolfgang W. Schmahl, Adrian Immenhauser
Chemical Geology 482 (2018) 72–90
DOI: 10.1016/j.chemgeo.2018.01.035