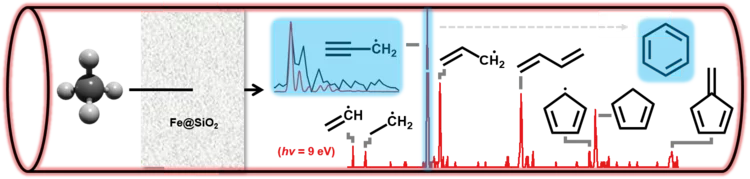
Watch them growing: New mechanistic insights into catalytic methane coupling
Methane valorization is a promising technology to utilize this platform compound to produce aromatics and hydrocarbons. Researchers from PSI and ETH Zürich unveiled this reaction mechanism and observed the molecular growth from the ground up. Besides stepwise CH3 addition, novel routes involving the dimerization of resonantly stabilized propargyl (C3H3) radicals to benzene (C6H6) were identified. These mechanistic insights will aid the development of valorisation strategies.
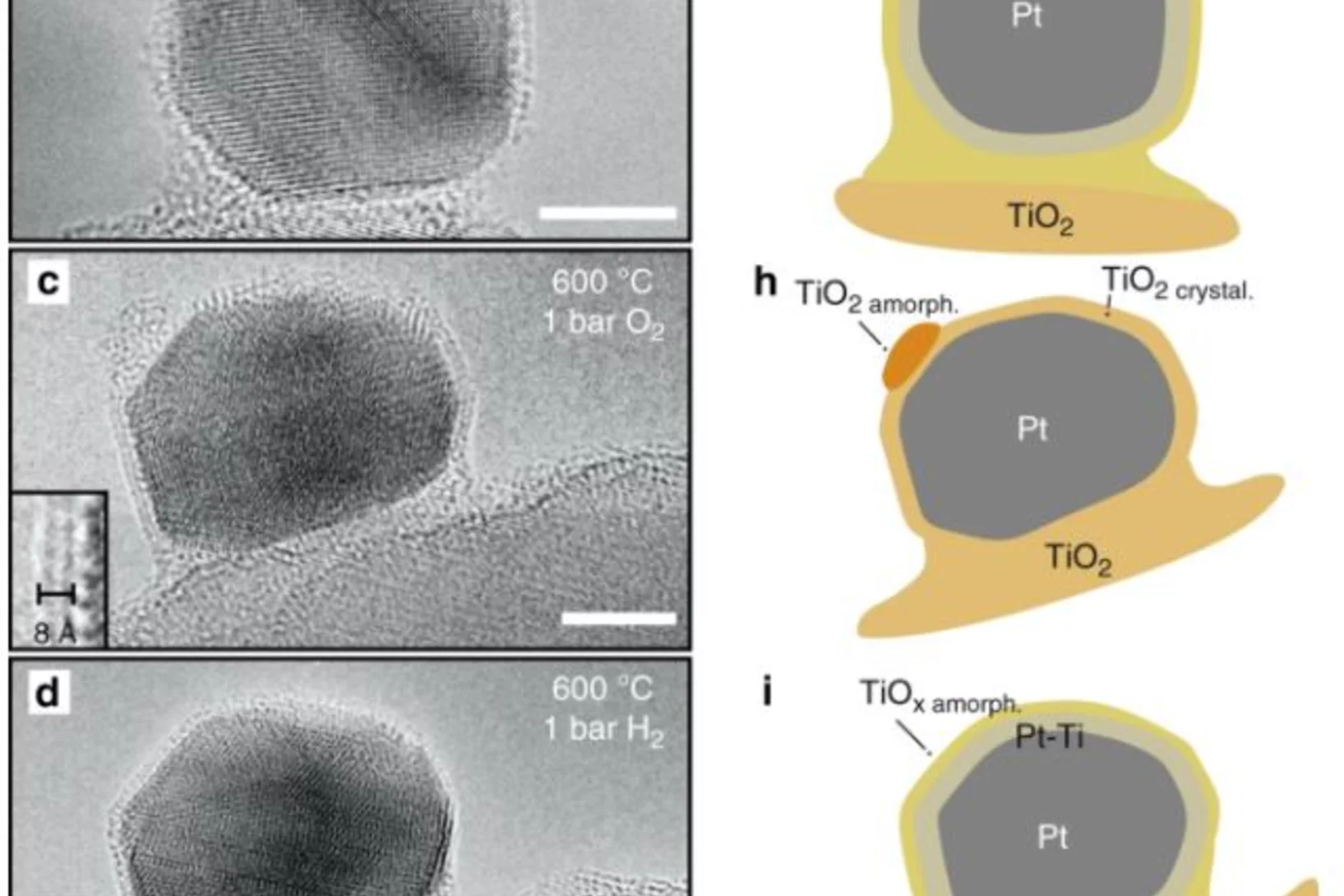
The dynamics of overlayer formation on catalyst nanoparticles and strong metal-support interaction
The editors at Nature Communications have put together an Editors’ Highlights webpage of recent research called “Catalysis” and chose to feature Arik Beck's et al. article, entitled “The dynamics of overlayer formation on catalyst nanoparticles and strong metal-support interaction”.
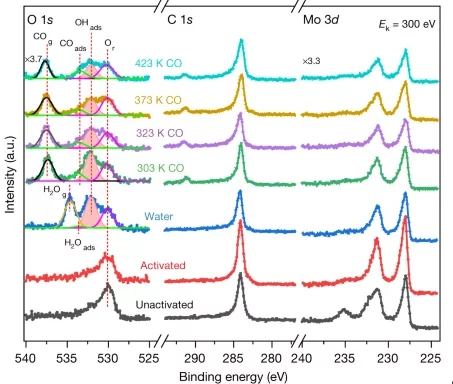
PUBLISHED IN NATURE: A stable low-temperature H2-production catalyst by crowding Pt on α-MoC
Platinum isolated atoms and clusters supported on molybdenum carbide have been extensively characterized. The presence of both species is essential to boost the stability, so that the catalysts displays high metal-normalized turnover number of 4,300,000 moles of hydrogen per mole of platinum
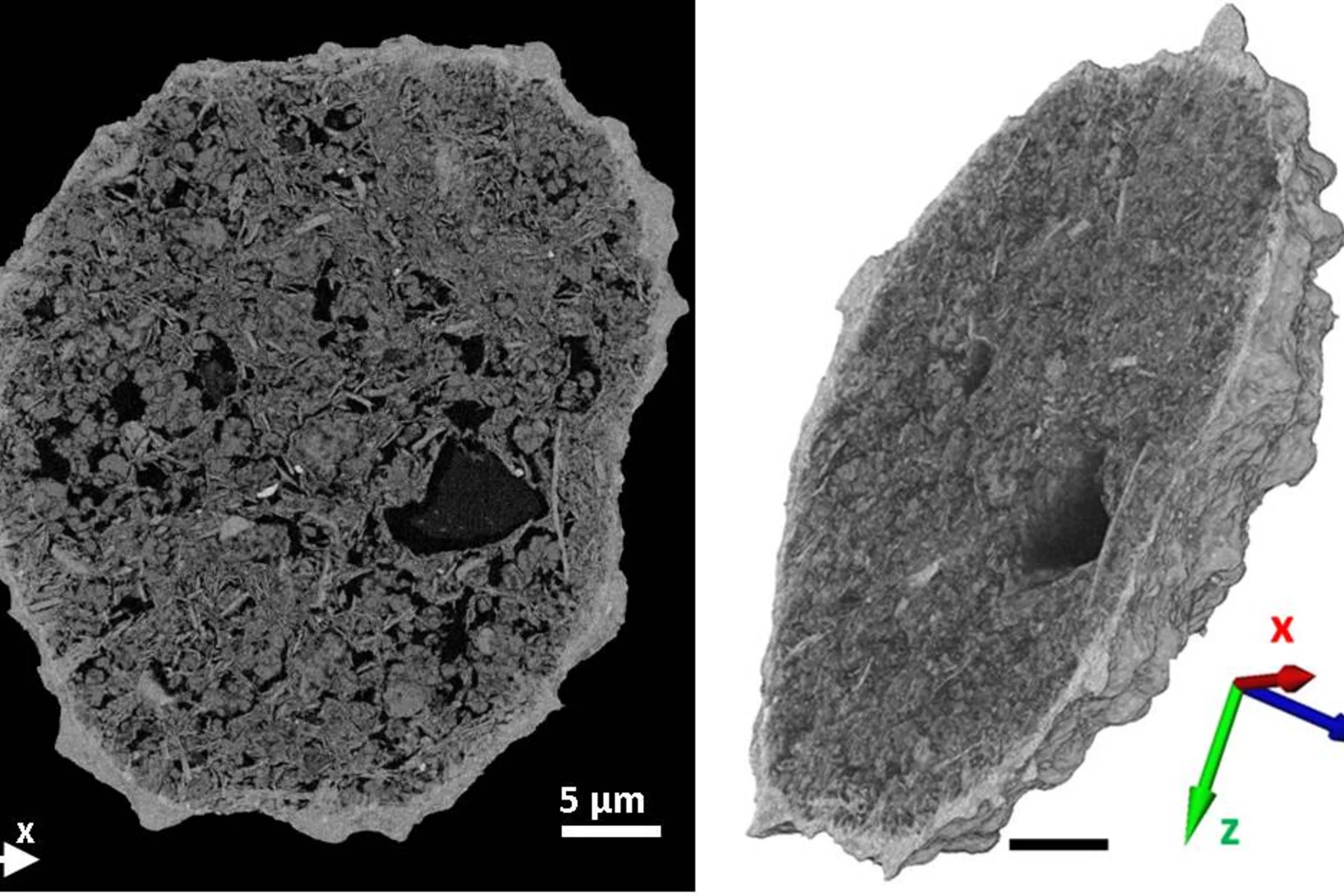
How to trick electrons to see the hidden face of crystals - LSK researchers try a trick for complete 3D analysis of submicron crystals
Breakthrough in 3D structure analysis by transmission electron microscopy (TEM) achieved by members of the LSK. Their paper published in Nature Communications will add a new dimension to TEM and thus is highlighted by Die Presse, Der Standard and phys.org.
Floating islands to convert sunlight into energy
A recent paper published in PNAS and highlighted by CNN proposes creating islands to produce a liquid fuel (methanol) by recycling atmospheric CO2.
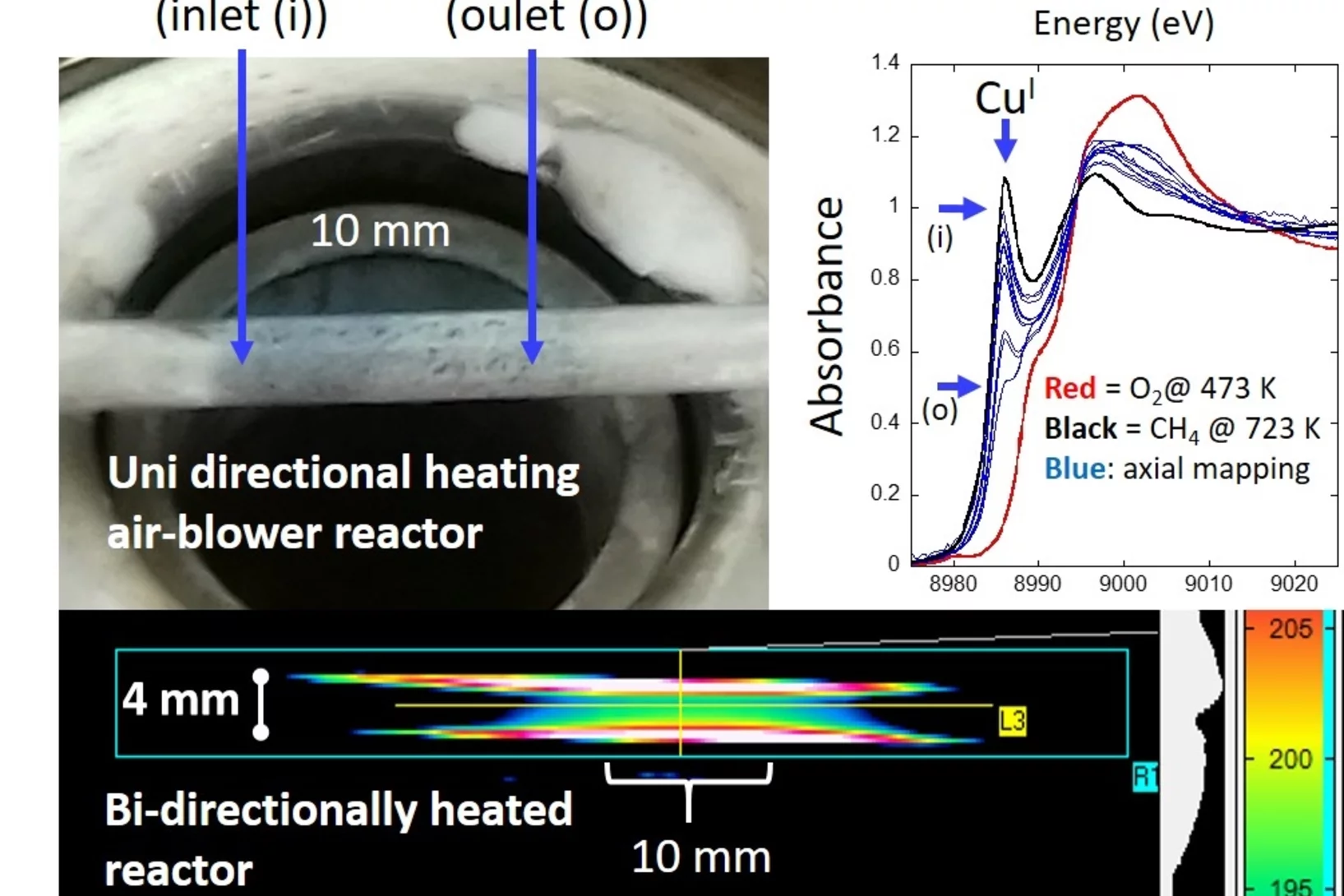
On isothermality in some commonly used plug flow reactors for X-ray based investigations of catalysts
Understand reactor design and characterize their behavior to avoid unwanted sources of error in determining structure function relationships in catalysis.
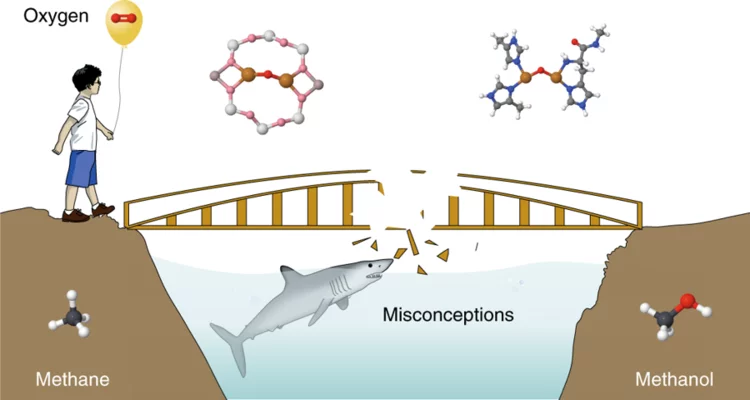
Article published in Nature Catalysis - view on misconceptions and challenges in methane-to-methanol
Manoj Ravi, PhD student in the van Bokhoven group, warned the community about all the misconceptions and pitfalls he encountered while studying the conversion of methane to methanol and made it into Nature Catalysis!
ESRF Scientific Highlight 2018
Our work on active site structure in mordenite has been featured as one of the Scientific Highlights of 2018 by the European Synchrotron Radiation Facility (ESRF).
BREAKTHROUGH
Recent advances in electron crystallography rank 5th breakthrough of the year 2018
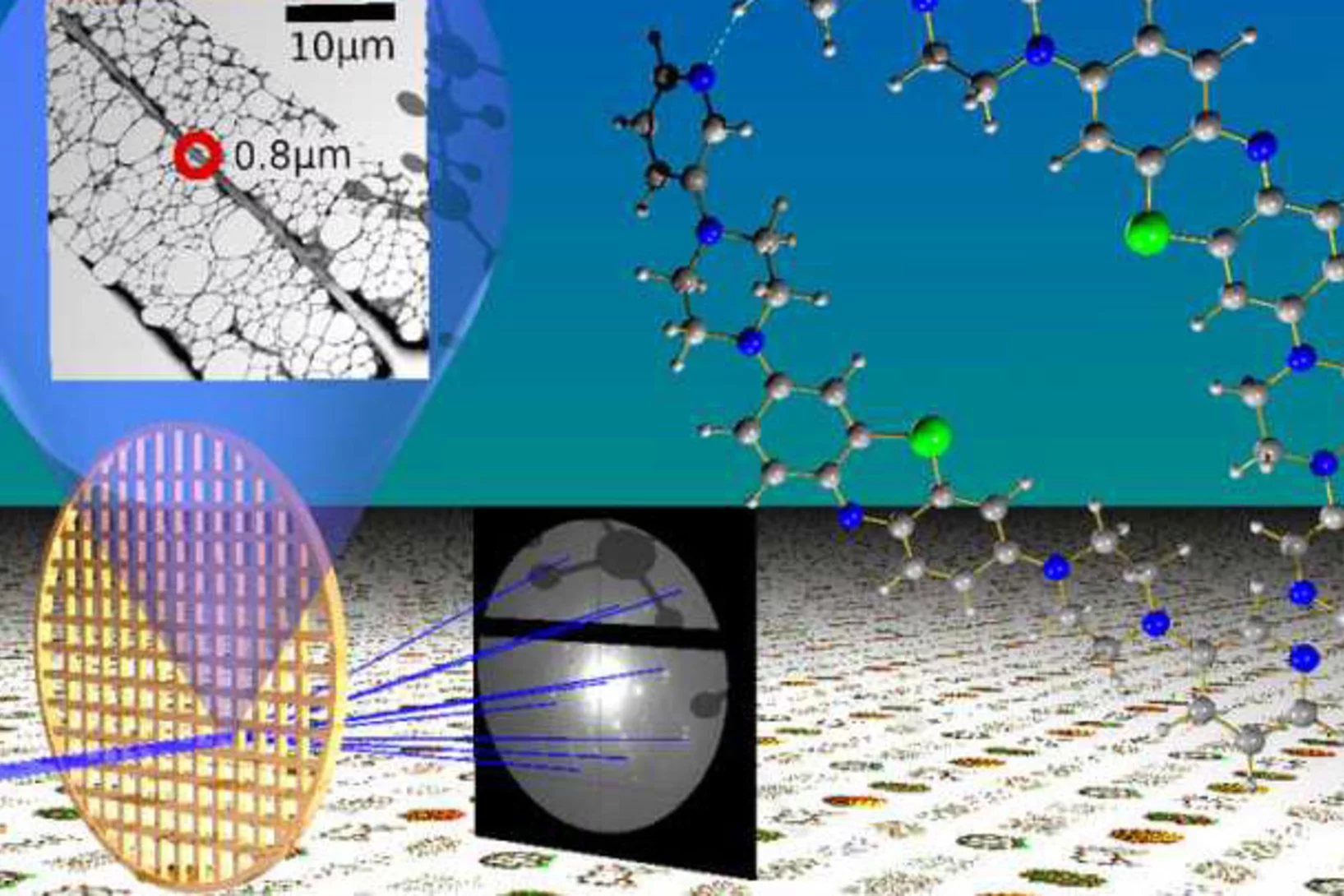
Breakthrough - electron crystallography for everyone
Recent advances in electron crystallography published in Angewandte Chemie and highlighted by Science, Chemical & Engineering News and ScienceNews!Under the lead of LSK member, "Rapid structure determination of microcrystalline molecular compounds using electron diffraction", published in Angewandte Chemie International Edition https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201811318 has attracted great attention in the chemistry community.
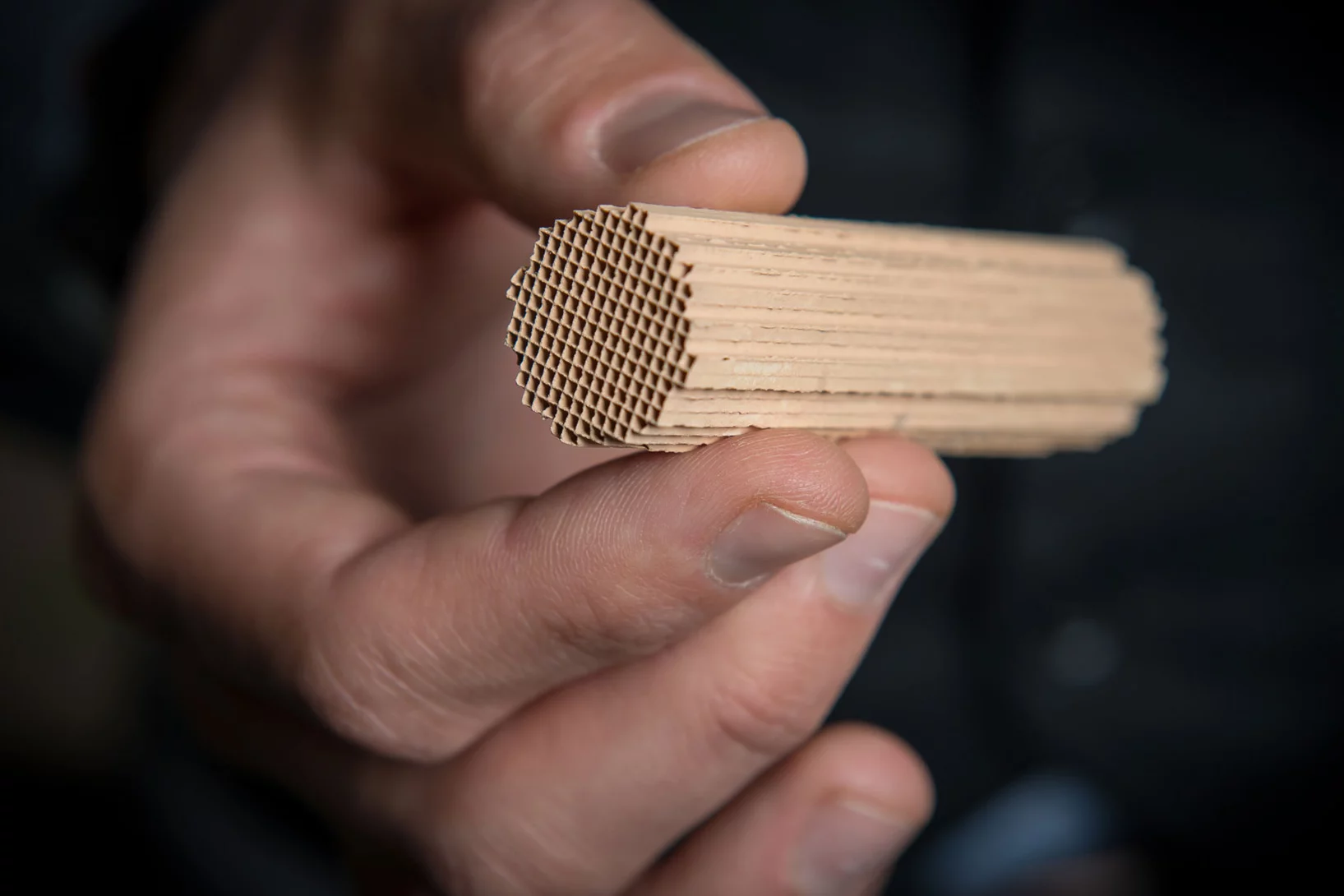
Gaz d'échappement purifiés grâce à une structure en éponge
Des chercheurs du PSI ont développé un nouveau catalyseur pour le traitement des gaz d'échappement des moteurs à gaz naturel. Il reste très actif même à basse température et pendant longtemps. La combustion du gaz naturel est ainsi plus propre et plus respectueuse du climat. Cela renforce l'attrait du gaz naturel et du biogaz comme alternative aux produits pétroliers, par exemple comme carburant pour les voitures.
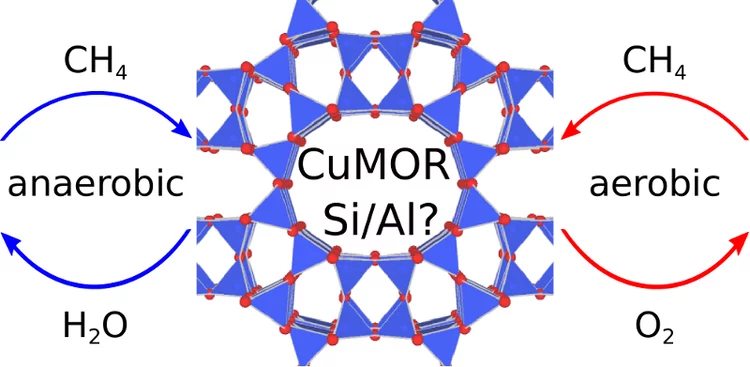
A new paper by Vitaly Sushkevich and Dennis Palagin is accepted to Angewandte Chemie
A new paper by Vitaly Sushkevich and Dennis Palagin is accepted to Angewandte ChemieNew issue of Angewandte Chemie is featuring a paper entitled "Effect of Active Sites Structure on Activity of Copper Mordenite in Aerobic and Anaerobic Conversion of Methane to Methanol" by Vitaly and Dennis.
For the second time on the cover of Journal of Physical Chemistry
For the second time on the cover page of Journal of Physical Chemistry.
Johannes Ihli's article makes it into ESRF Highlights
ESRF Highlights 2017 features work of researchers from the Laboratory of Catalysis and Sustainable Chemistry, the Photon Science Division at Paul Scherrer Institut and the ESRF, Grenoble, France.
Johannes Ihli and co-researchers made it into Nature Communications
Nature Communications will be publishing the paper of Johannes Ihli et al., "A three-dimensional view of structural changes caused by deactivation of fluid catalytic cracking catalysts", in issue 8, article number 809. The paper has already gone online on October 9, 2017.
Cover page of The Journal of Physical Chemistry
The paper titled "Core–Shell Structure of Palladium Hydride Nanoparticles Revealed by Combined X-ray Absorption Spectroscopy and X-ray Diffraction" by Aram L. Bugaev was published in The Journal of Physical Chemistry and made it on the cover of issue 33/2017.
SPS Award in Applied Physics awarded to Waiz Karim
The SPS 2017 Prize in Applied Physics is awarded to former member of the van Bokhoven Group Waiz Karim for his PhD thesis entitled "Metal nanostructures and their catalytic properties using top-down nanofabrication and single particle spectroscopy" which was honored with his 1st author publication "Catalyst support effects on hydrogen spillover" by W. Karim, C. Spreafico, A. Kleibert, J. Gobrecht, J. VandeVondele, Y. Ekinci, J. A. van Bokhoven, Nature, 2017, 541, 68–71.
Rendre une précieuse matière première exploitable grâce à l’eau
Lors de l’extraction du pétrole, on se contente le plus souvent de brûler le méthane, alors qu’il pourrait utilement servir de matière première à la fabrication de certains carburants et produits de l’industrie chimique. Un moyen de rendre le méthane exploitable consiste à le transformer en méthanol. Des chercheurs de l’Institut Paul Scherrer PSI et de l’ETH Zurich ont récemment développé un procédé qui permet de réaliser efficacement cette conversion à faible coût.
Cover page of CHEMCATCHEM
A paper titled "Optimization of the Reaction Conditions for Catalytic Fast Pyrolysis of Pretreated Lignin over Zeolite for the Production of Phenol" by Zhiqiang Ma is published in ChemCatChem and made it on the cover of issue 6/2017.
De nouvelles approches des réactions chimiques grâce aux nanotechnologies
80 % des produits de l’industrie chimique sont fabriqués par recours à la catalyse. Ce procédé est également indispensable dans la conversion énergétique et l’épuration des gaz d’échappement. L’industrie teste donc continuellement de nouvelles substances et de nouvelles configurations susceptibles de déboucher sur de nouveaux procédés catalytiques plus performants. Des chercheurs de l’Institut Paul Scherrer PSI à Villigen et de l’ETH Zurich ont à présent développé une méthode qui permet d’améliorer nettement la précision de tels essais, ce qui devrait accélérer la recherche de solutions optimales.
Exploiter le méthane au lieu de le brûler à la torche
Des chimistes à l’ETH Zurich et à l’Institut Paul Scherrer ont découvert un nouveau moyen direct pour transformer le méthane gazeux en méthanol liquide. A l’avenir, cela pourrait inciter l’industrie à exploiter davantage ce gaz au lieu de le brûler sans l’utiliser, comme elle le fait aujourd’hui.
Textbook on XAS and XES
During the last two decades, remarkable and often spectacular progress has been made in the methodological and instrumental aspects of x–ray absorption and emission spectroscopy. This progress includes considerable technological improvements in the design and production of detectors especially with the development and expansion of large-scale synchrotron reactors All this has resulted in improved analytical performance and new applications, as well as in the perspective of a dramatic enhancement in the potential of x–ray based analysis techniques for the near future.
Controlling tunnelling in methane loss from acetone ions by deuteration
If a ball is rolled up a hill with less kinetic energy than the potential energy at the top, it will return eventually, and stays bound in the valley. Tunnelling is a distinctly quantum mechanical phenomenon, in which such balls can magically cross the hill, and appear in the neighbouring valley, as if going through a tunnel. In order for this to happen with a non-negligible probability, the ball has to be small and the barrier, i.e. the hill, sharp.
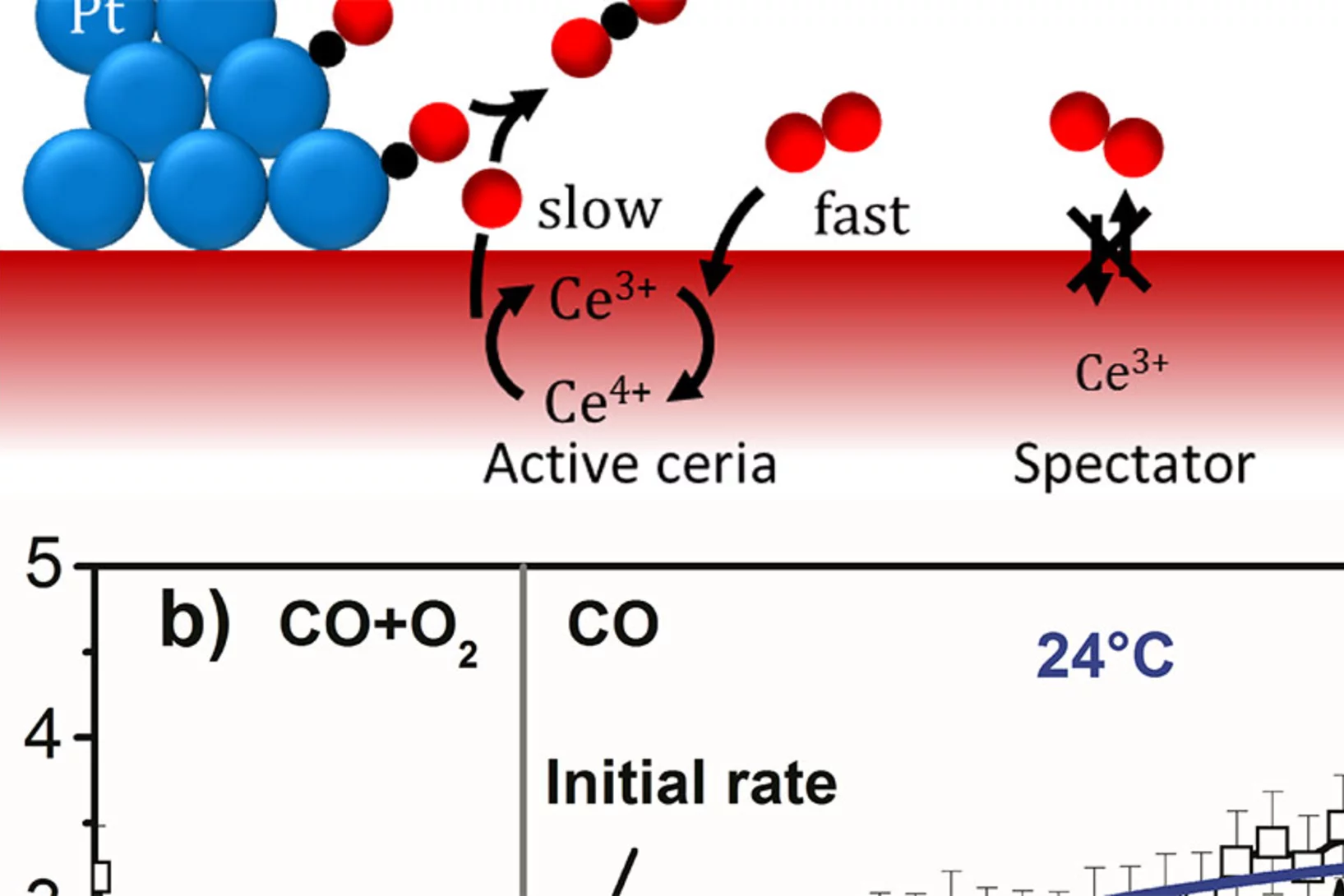
Catalytically Active and Spectator Ce3+ in Ceria-Supported Metal Catalysts
Identification of active species and the rate-determining reaction steps are crucial for optimizing the performance of oxygen-storage materials, which play an important role in catalysts lowering automotive emissions, as electrode materials for fuel cells, and as antioxidants in biomedicine. We demonstrated that active Ce3+ species in a ceria-supported platinum catalyst during CO oxidation are short-lived and therefore cannot be observed under steady-state conditions.
Metal organic frameworks for photo-catalytic water splitting
Growing experimental and computational evidence suggests that metal organic frameworks (MOFs) can make a meaningful contribution to catalytically promoted water splitting. They offer an impressive physical, spatial, chemical and electronic mutability with which to support and sustain water splitting half reactions. Their classical features à thermal stability, large surface area, high porosity and modularity à define them as versatile solid supports.
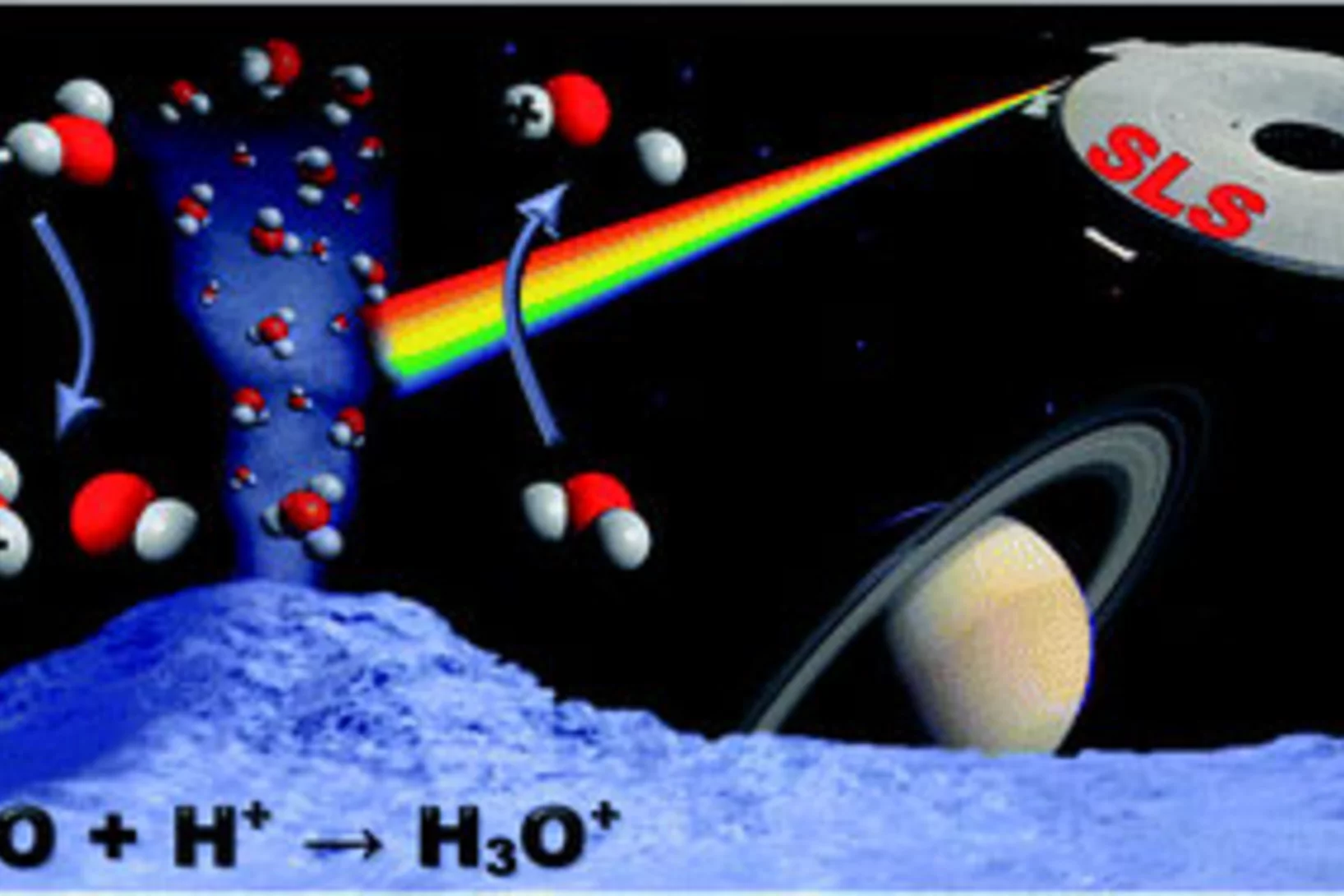
On the protonation of water
Imaging photoelectron photoion coincidence (iPEPICO) spectroscopy on isolated water molecules and water dimers establishes a new route to determining the water proton affinity (PA) with unprecedented accuracy. A floating thermochemical cycle constructed from the OH+ and H3O+ appearance energies and three other spectroscopic values establishes the water PA as 683.22 ± 0.25 kJ mol−1 at 0 K, which converts to 688.81 ± 0.25 kJ mol−1 at room temperature.
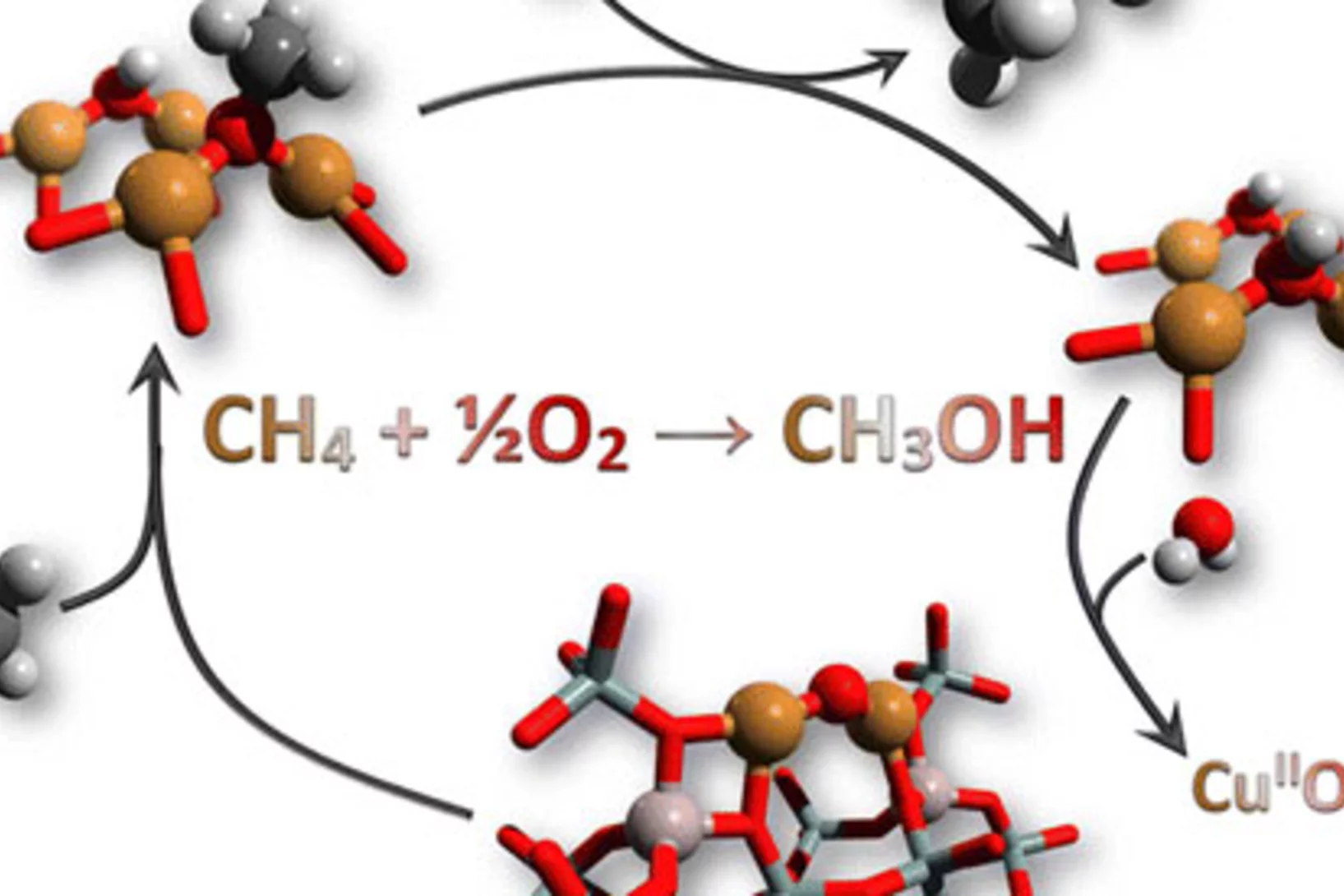
Reaction Conditions of Methane-to-Methanol Conversion Affect the Structure of Active Copper Sites
Determining the structure of the active Cu sites, which are associated with the methane conversion intermediate during stepwise, low-temperature, methane-to-methanol conversion, represents an important step for the upgrade of this reaction route to a viable process. Quick X-ray absorption spectroscopy allowed us to follow the electronic and structural changes to the active Cu sites during reaction with methane and during desorption of the activated intermediate. A large fraction (41%) of the oxygen-activated CuII reacted with methane and underwent reduction to CuI.