The group ‘in-situ spectroscopy for Environmental Science’ operates the PHOENIX (PHotons for the Exploration of Nature by Imaging and XAFS) beamline. PHOENIX is one of the few undulator beamlines worldwide, which offer x-ray microspectroscopy for both soft x-rays and the rarely served tender energy range. The scientific goal of the beamline is the in situ characterization of crystalline and amorphous materials in terms of morphology, atomic composition, local order, ligand coordination, and electronic structure at different time and length scales ranging from a few mm to a few micrometer. The energy range ($0.3-8$~keV) covers the K-absorption edges of low Z-elements (O to Fe) and important L-edges (Ca to La), which places the PHOENIX team in a unique position to address important questions related to environmental science, catalysis, energy research, earth science, biology, geochemistry and the design of new functional materials. As undulator beamline PHOENIX offers flux-hungry techniques, in particular x-ray absorption and emission microspectroscopy (XAS and XES), for dilute systems as well as scanning fluorescence microscopy with 3 micron spatial resolution.
A major part of the activities of the PHOENIX team are devoted to instrumental developments, most notably the implementation of emission spectroscopy for the tender X-ray range with a new von Hamos spectrometer. Furthermore, in a collaborative effort with Dr. G. Smolentsev, novel pump and probe schemes for tender X-rays are currently under development and this experiment is currently open for first experienced users for pilot experiments. Duetto and IDOL lasers Duetto and IDOL lasers are available for photoexitation.
The group also develops microfluidic devices for the energy range available at PHOENIX, enabling transmission and fluorescence type XAS measurements for in-house and user experiments. The devices have flexible design for studying single or multiple reactant solutions, offering precise control, rapid mixing and the possibility to transpose a chemical reaction onto spatial coordinates.
The group's own research program aims to study in situ processes relevant to environmental sciences and energy research. Currently, our research aims to study the first steps of crystallization processes using liquid cells and a liquid microjet technique.
Scientific Highlights
Aerosol-based synthesis of pure and stable amorphous calcium carbonate
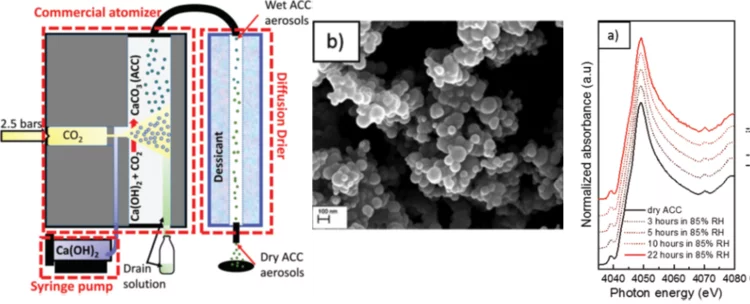
Calcium carbonates are key materials to biomineralization, they are frequently used in industrial applications and also for carbon capture technologies. Finally, they serve as an important model system to test novel nucleation theories. Calcium carbonate crystalizes in a multi-step process, where amorphous calcium carbonate (ACC) is the most important precursor in the crystallization process. Existing synthesis protocols generate ACC of different stability and purity. To improve our mechanistic understanding of carbonate crystallization, reactivity and polymorph formation, the reproducible synthesis of clean and stable ACC is an important, and yet unresolved step. Here we use the fast reaction of CO2 with calcium hydroxide in airborne aerosols to reproducibly create pure and stable ACC, which may serve as a well-defined starting material for further chemical processing.
Tuning the magnesium content in magnesium rich-calcites
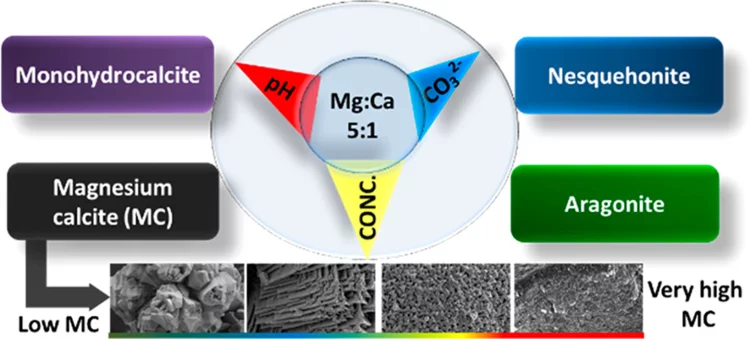
Magnesium rich calcites are important functional biominerals. For example, they can be found in protective shells or eye lenses. Natural organism provide a surprisingly high degree of control on the amount of magnesium incorporation into calcites by yet not well understood mechanisms. Understanding such control mechanism is important when designing bio inspired functional materials. Here we systematically explore the impact of thermodynamic parameters on the degree of magnesium incorporation into calcite. In particular, we identify the thermodynamic conditions, where very high magnesium rich calcites (50% Mg/50% Ca) forms under ambient conditions of temperature and pressure. This is an important finding for geochemistry: Very high magnesium rich calcite is believed to be the precursor for dolomite. Despite its frequent occurrence in nature, its unknown formation pathway remains one of the big mysteries in geochemistry.
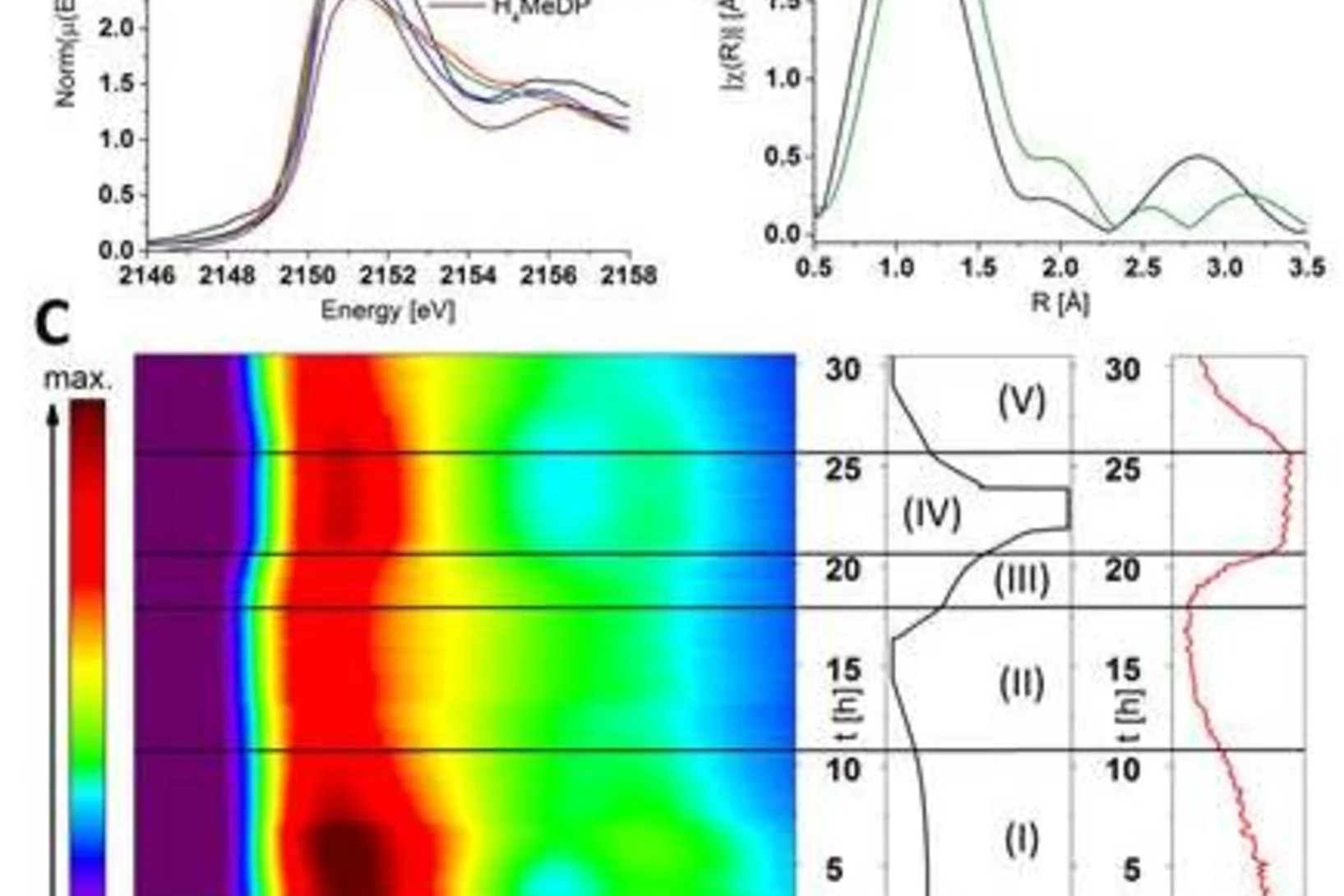
Investigation of anionic redox activities in organic-based electrode for Li-ion batteries
To date the electrochemical activity of battery materials was always relying in the oxidation/reduction of cationic redox (change of oxidation state of transition metals generally). However, recently, it was established in new cathode materials (so call Li-rich cathode) that the oxygen from the crystal lattice might also play the role of anionic redox center leading to enhance then the specific charge of battery materials.