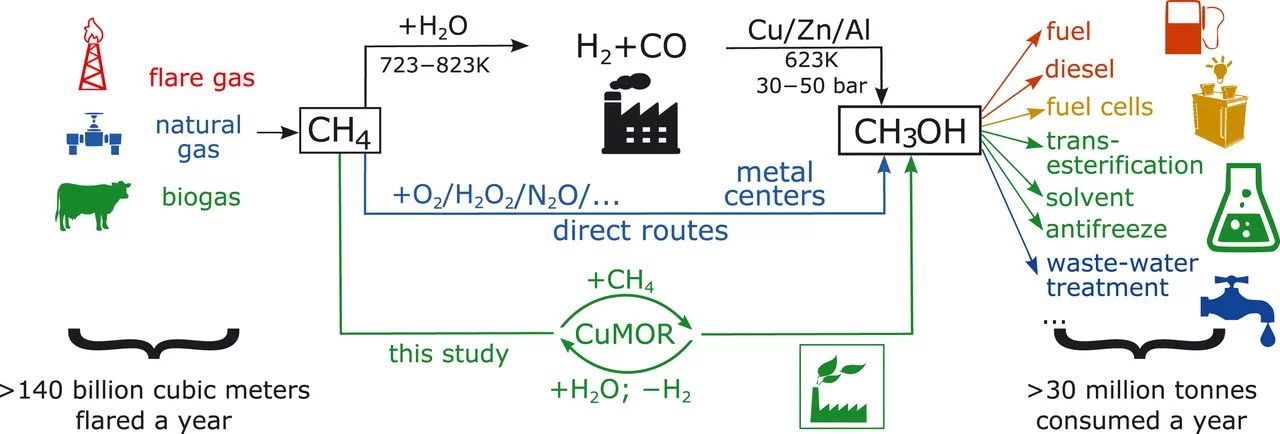
direct functionalization of methane in natural gas remains a key challenge. We present a direct stepwise method for converting methane into methanol with high selectivity (~97%) over a copper-containing zeolite, based on partial oxidation with water. The activation in helium at 673 kelvin (K), followed by consecutive catalyst exposures to 7 bars of methane and then water at 473 K, consistently produced 0.204 mole of CH3OH per mole of copper in zeolite. Isotopic labeling confirmed water as the source of oxygen to regenerate the zeolite active centers and renders methanol desorption energetically favorable. On the basis of in situ x-ray absorption spectroscopy, infrared spectroscopy, and density functional theory calculations, we propose a mechanism involving methane oxidation at Cu II oxide active centers, followed by Cu I reoxidation by water with concurrent formation of hydrogen.
Contact
Dr Maarten NachtegaalSuperXAS beamline
Laboratory for Synchrotron Radiation and Femtochemistry (LSF)
Swiss Light Source, Paul Scherrer Intitute
5232 Villigen-PSI, Switzerland
Telephone: +41 56 310 30 56
E-mail: marten.nachtegaal@psi.ch
Original Publication
Selective anaerobic oxidation of methane enables direct synthesis of methanolVitaly L. Sushkevich, Dennis Palagin, Marco Ranocchiari, Jeroen A. van Bokhoven
Science, 5 May 2017
DOI: 10.1126/science.aam9035