Operando X-ray Tomographic Microscopy of Polymer Electrolyte Fuel Cell Freeze Start
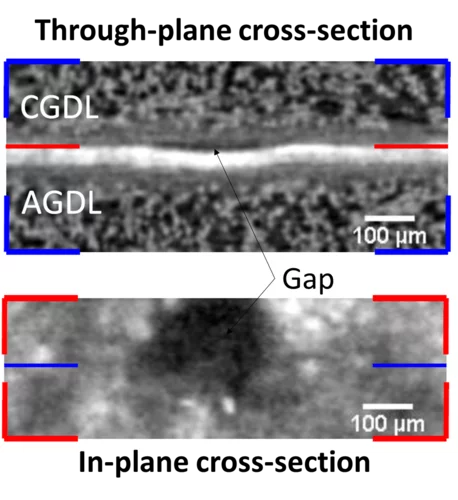
Understanding the water management in polymer electrolyte fuel cells (PEFCs) during sub-zero operation is crucial for designing effective freeze start strategies. In collaboration with Toyota Motor Europe sub-second X-ray tomographic microscopy was used to study the water distributions in the gas diffusion layer (GDL) of PEFCs during dynamic freeze starts from −30 °C that mimic automotive freeze start conditions at different pre-drying levels and varying the feed gas humidity.
Direct evidence of in situ Co-oxyhydroxide formation on the surface of La0.2Sr0.8CoO3-δ water splitting catalyst
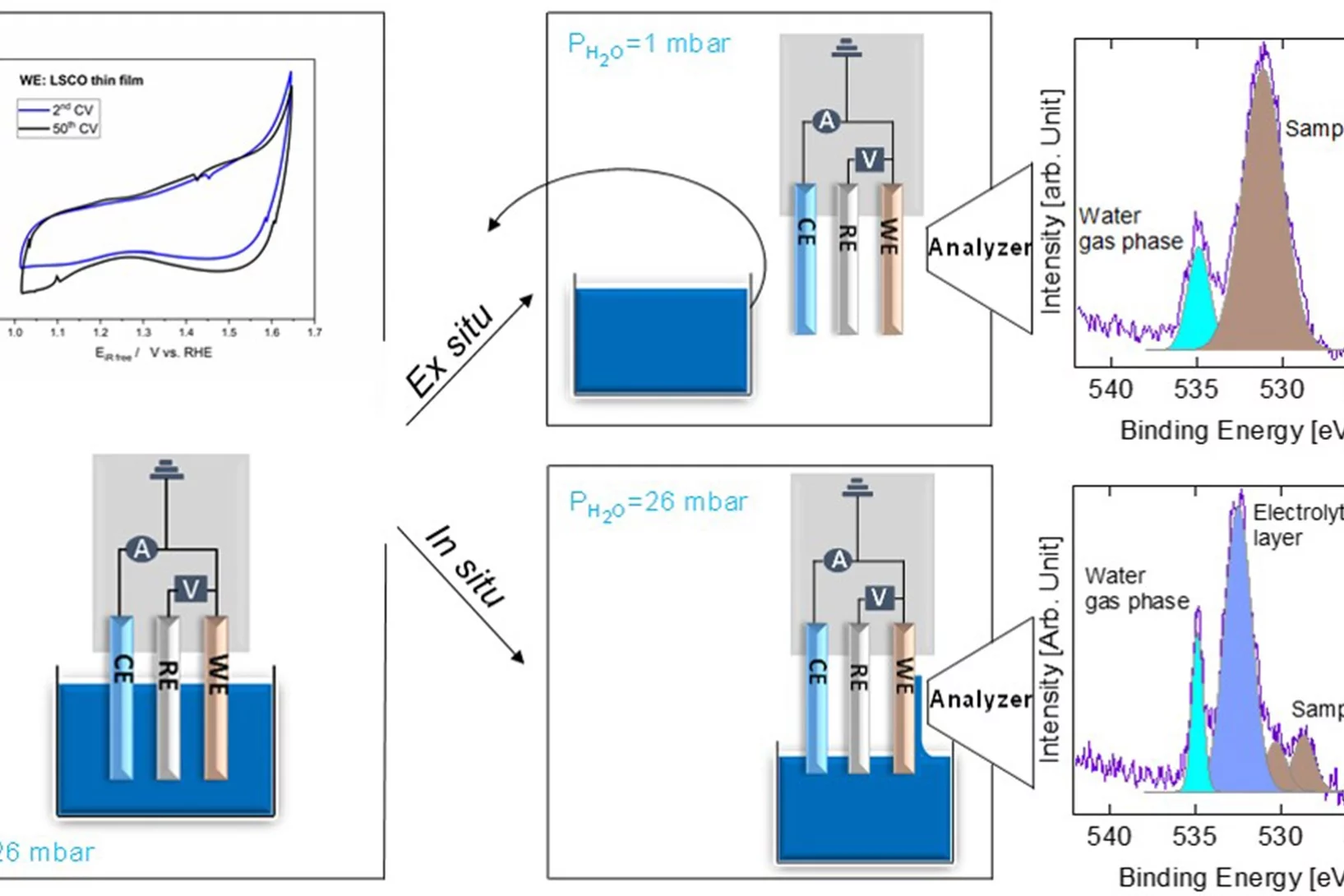
We carried out in situ and ex situ ambient pressure X-ray photoelectron spectroscopy (APXPS) experiments on a La0.2Sr0.8CoO3-δ perovskite oxygen evolution reaction (OER) catalyst. The study shows that Sr is leached into the electrolyte after immersion, leading to surface Co active site enrichment. Such a Co-enriched surface evolves into a new phase during operation. With the help of theoretical simulations, such a species is assigned to Co-oxyhydroxide, providing direct evidence of its formation during the OER.
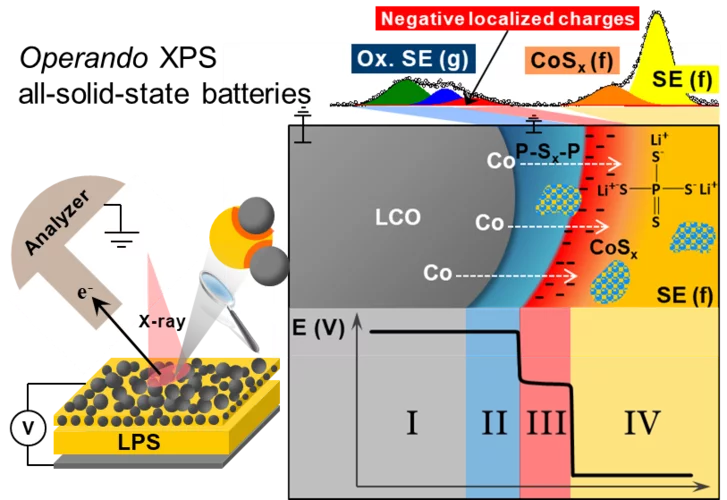
Reactivity and potential profile across the electrified LiCoO2-Li3PS4 interface probed by operando X-ray photoelectron spectroscopy
All-solid-state lithium batteries are a promising alternative for next generation of safe energy storage devices, provided that parasitic side reactions and the resulting hindrances in ionic transport at the electrolyte-electrode interface can be overcome. Motivated by the need for a fundamental understanding of such interface, we present here real-time measurements of the (electro-)chemical reactivity and local surface potential at the electrified interface Li3PS4 and LiCoO2 using operando X-ray photoelectron spectroscopy.
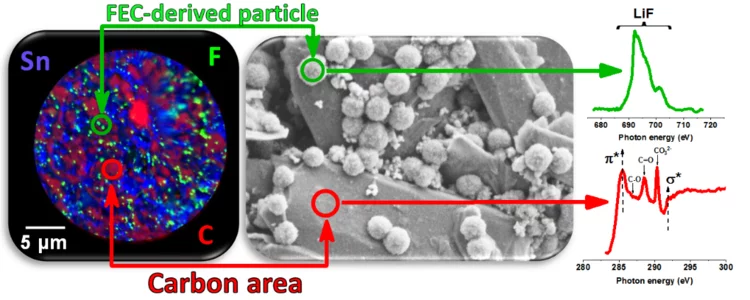
Deciphering the Mechanism of FEC-induced SEI Formation in Li-ion Batteries
Fluoroethylene-carbonate is often referred to as a film-forming electrolyte additive for Li-ion batteries, resulting in high quality Solid–Electrolyte-Interphase on negative electrode, however, the underlying mechanism, even if thought to be known, has been only clarified due to our targeted experimental design, combining systematic electrochemical, chemical and microscopy characterization techniques. We have shown that first the formation of inorganic LiF-rich particles appear and only later the carbonate-rich film is actually formed.
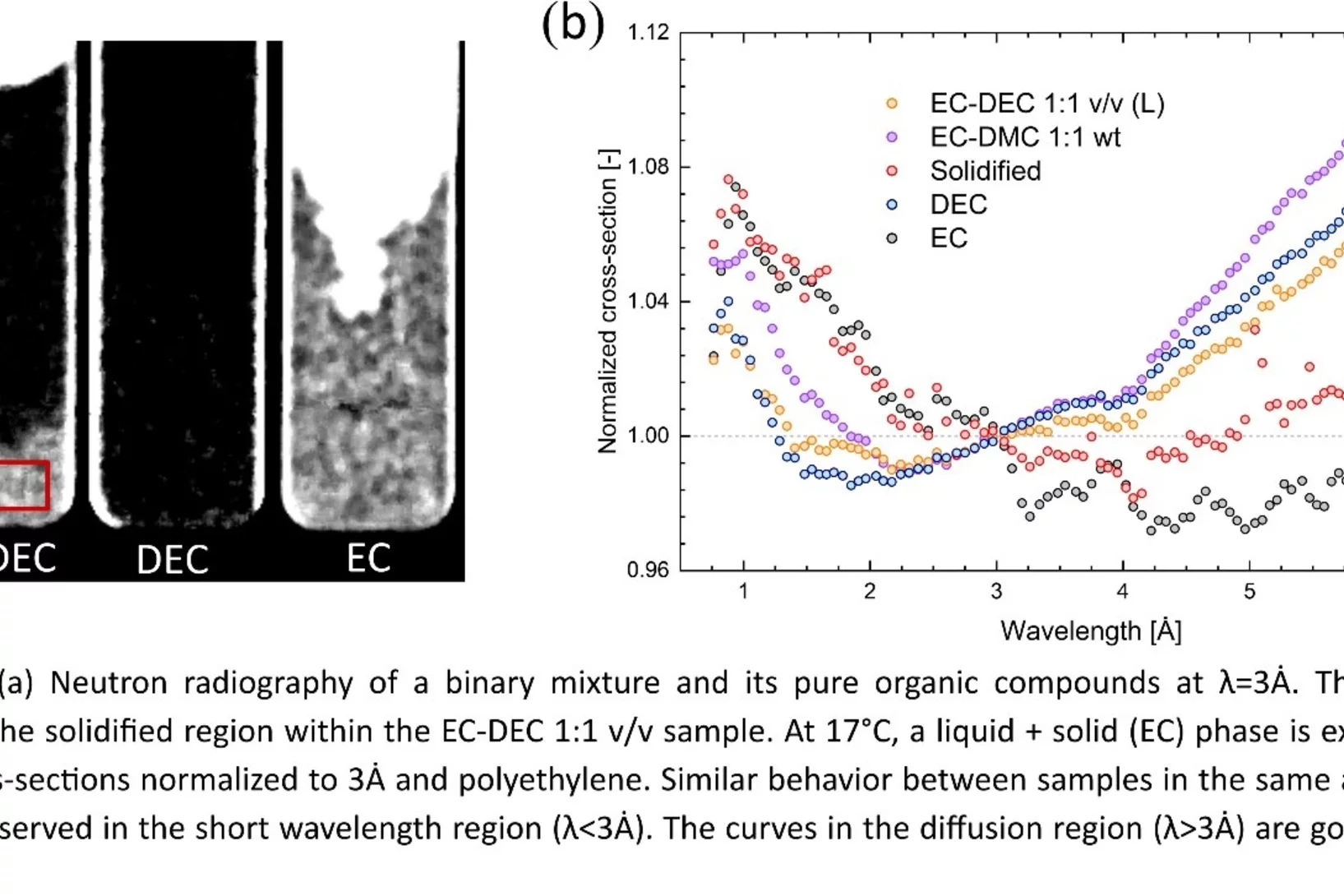
Towards in situ imaging of electrolyte physical and chemical changes in Li-ion batteries
The Lithium conducting electrolyte is a critical component of Li-ion batteries, as it has an important impact on performance and its durability, in particular in case of extreme environmental conditions. At low temperatures, a partial solidification can occur, while high temperatures can promote the degradation of the electrolyte. Using time-of-flight (ToF) neutron radiography, the possibility of imaging such changes in a fully non-invasive manner was demonstrated for the first time.
First direct observation of the oxygen transport in polymer electrolyte water electrolysis
PSI researchers have developed a new methodology for studying the complex transport processes in polymer electrolyte water electrolysis (PEWE). Using advanced operando X-ray tomographic microscopy, we were able to observe for the first time the formation of oxygen pathways in the porous transport layer, in three dimensions. Understanding oxygen transport is crucial for improving PEWE technology and this work provides precious insights for the design of future, better-performing PEWE cells.
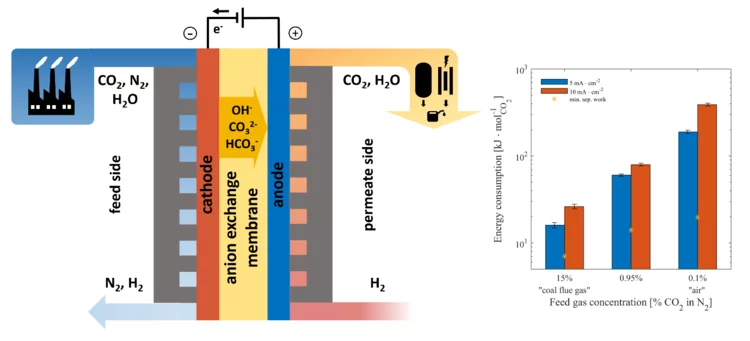
An electrochemical membrane processes for CO2 capture
CO2 capture from dilute gas mixtures (e.g., combustion flue gases, air) is increasingly recognized as a critical technological pathway towards stemming catastrophic climate change. Conventional thermal-based processes for removing CO2 from flue gas (e.g., amine scrubbing) are energy intensive and significantly reduce power plant efficiency. Electrochemical separation approaches have the potential to reduce these power requirements considerably by using electrons to transport CO2 as (bi)-carbonate ions across an alkaline membrane.
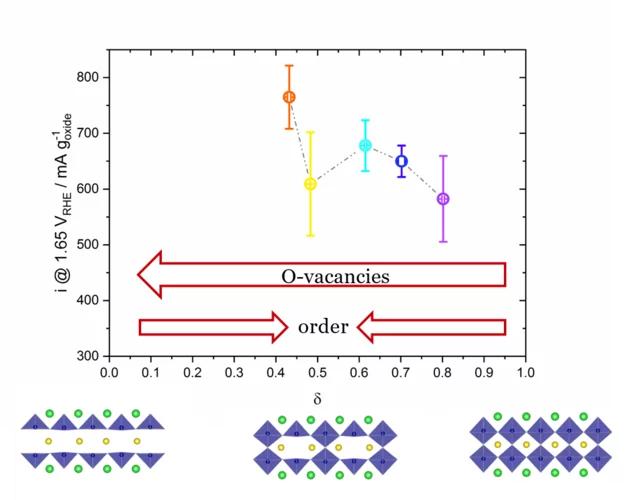
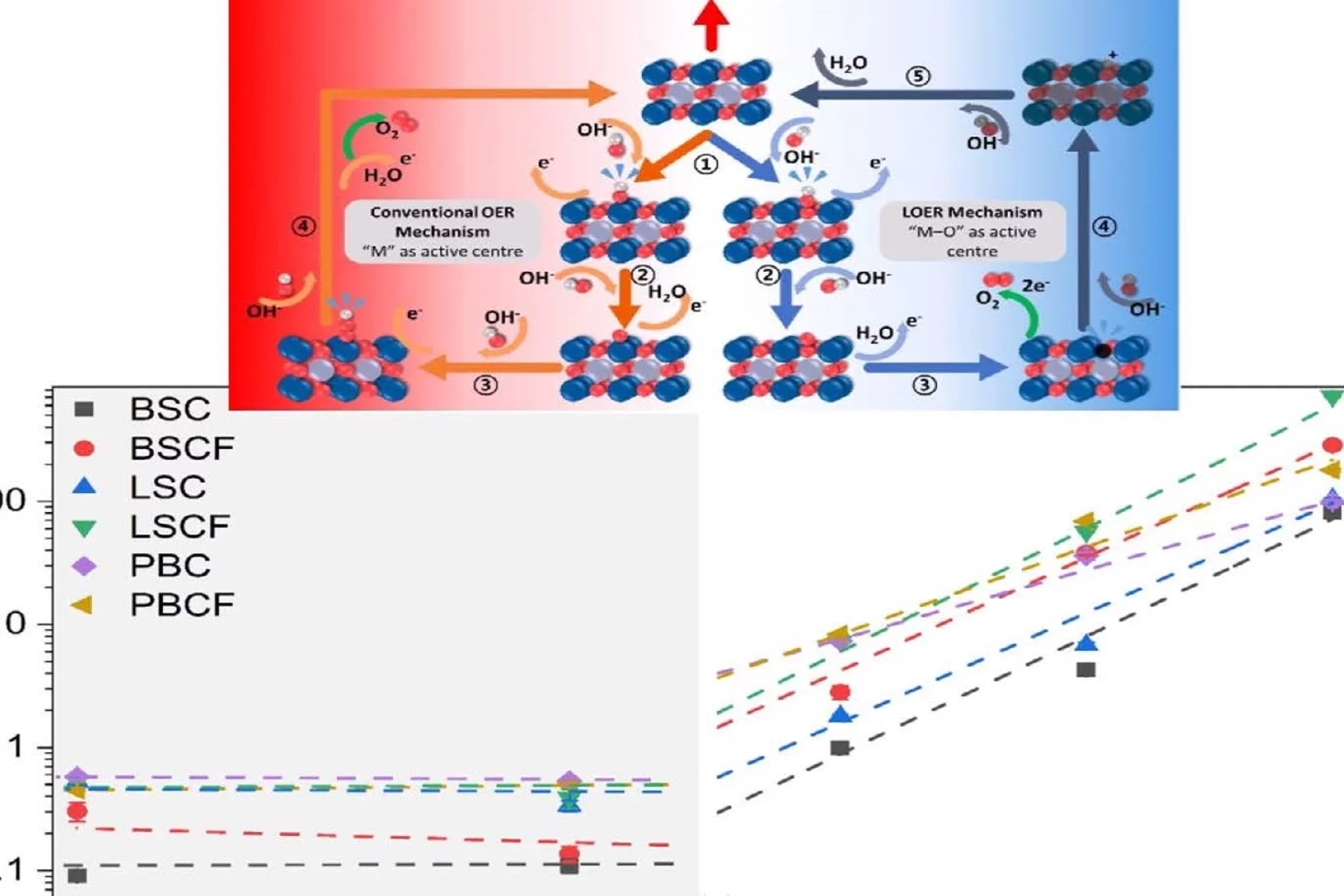
Correlation between Oxygen Vacancies and Oxygen Evolution Reaction Activity for a Model Electrode: PrBaCo2O5+δ
The role of the oxygen stoichiometry of perovskite catalysts in the oxygen evolution reaction (OER) is systematically studied in the PrBaCo2O5+δ family. The reduced number of physical/chemical variables combined with in-depth characterizations such as neutron diffraction, O K-edge X-ray absorption spectroscopy(XAS), electron energy loss spectroscopy (EELS), magnetization and scanning transmission electron microscopy (STEM) studies, helps investigating the complex correlation between OER activity and a single perovskite property, such as the oxygen content. Larger amount of oxygen vacancies appears to facilitate the OER, possibly contributing to the mechanism involving the oxidation of lattice oxygen, i.e., the lattice oxygen evolution reaction (LOER). Furthermore, not only the number of vacancies but also their local arrangement in the perovskite lattice influences the OER activity, with a clear drop for the more stable, ordered stoichiometry.
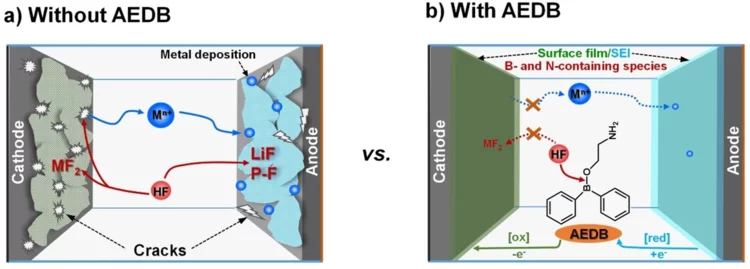
Cross-Talk–Suppressing Electrolyte Additive for Li-ion Batteries
Control of interfacial reactivity at high-voltage is a key to high-energy-density Li-ion batteries. 2-aminoethyldiphenyl borate was investigated as an electrolyte additive to stabilize surface and bulk of both NCM851005 and graphite in the cell with upper cut-off voltage of 4.4 V vs Li+/Li. AEDB almost completely eliminated the “cross-talk” in the cell, by significantly reducing metal leaching from the cathode, preventing their deposition at the anode, and further electrolyte decomposition.
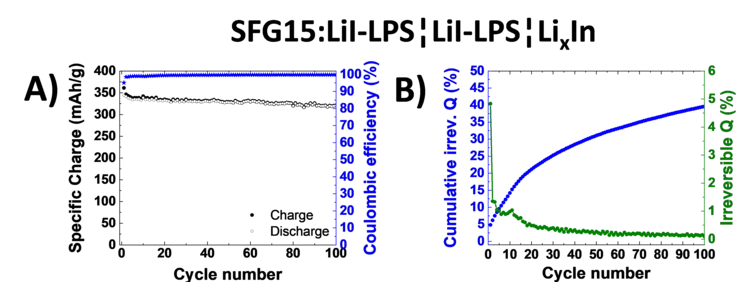
Excellent cycling stability of graphite in all-solid-state battery using sulfide solid electrolyte
All-solid-state lithium ion batteries represent a promising battery technology for boosting the volumetric energy density and promising a superior safety. In this study excellent cycling stability of graphite anode material have been demonstrated in combination with sulfide-based solid electrolyte. Furthermore we evaluated the stability of the graphite-electrolyte interface by analyzing the normalized cumulative irreversible charge during cycling experiments.
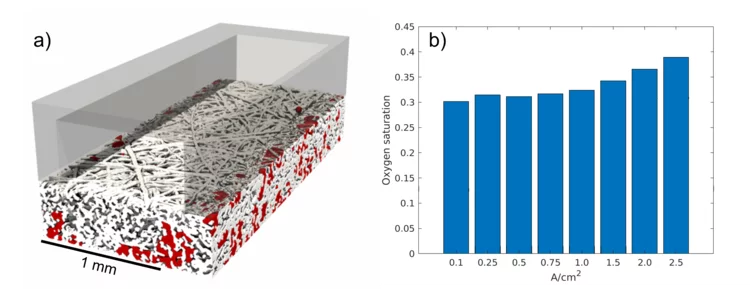
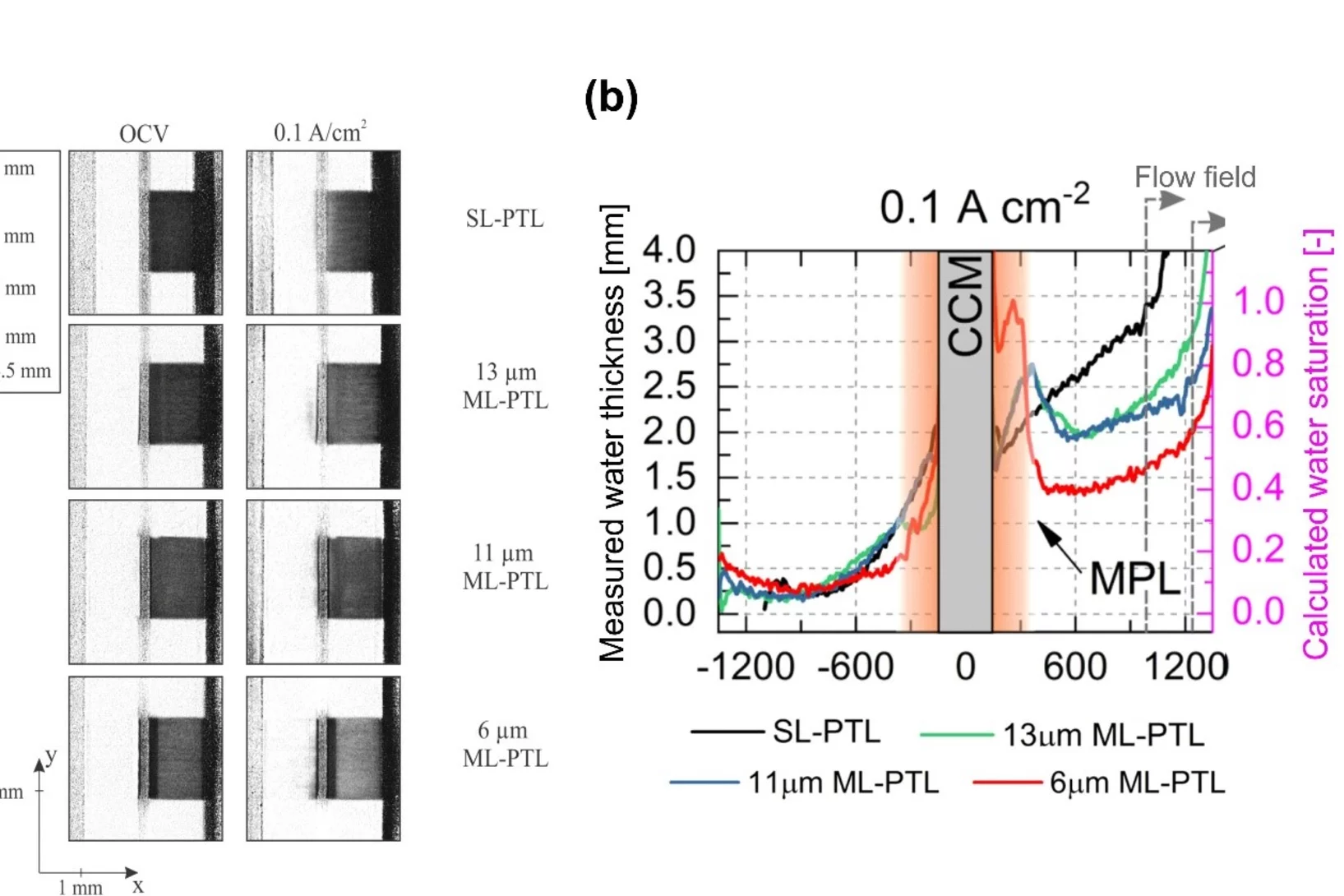
Impact of micro-porous layers (MPL) on two-phase flow in electrolyzers
Polymer Electrolyte Water Electrolyzers (PEWE), due to their excellent dynamic characteristics, can provide an economical solution to the intermittent nature of new renewable sources, by converting the excess electricity into hydrogen. However, improvements in efficiency and in capital cost are still required for the large-scale deployment of this solution. In this context, we studied whether the efficiency improvements observed when using porous structures featuring a micro-porous layer (MPL) can be attributed to a better distribution of the water.
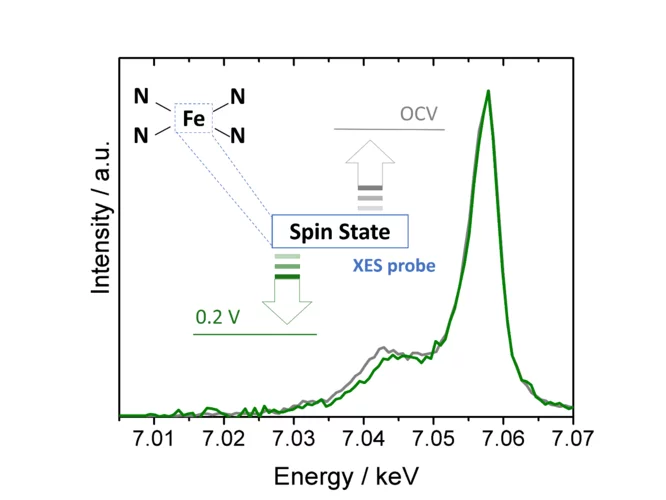
Using X-ray emission spectroscopy to study the electronic properties of single atom catalysts
Single atom catalysts hold great promise as O2- or CO2-reduction electrocatalysts, but a deeper understanding of their active sites’ structure and electronic properties is needed in order to render them sufficiently active and stable. To this end, we have used X-ray emission spectroscopy to determine these catalysts’ electronic configuration, and performed in situ measurements that unveil the effect of potential on this key feature.
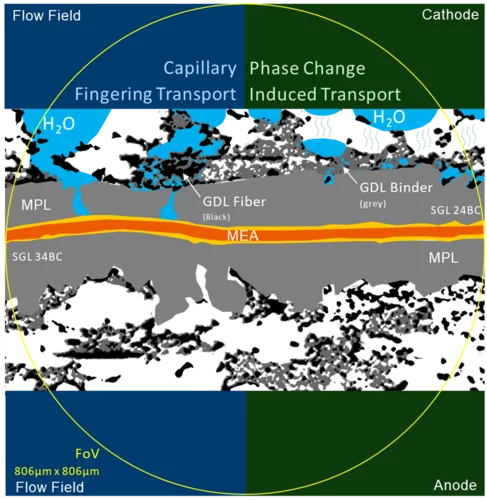
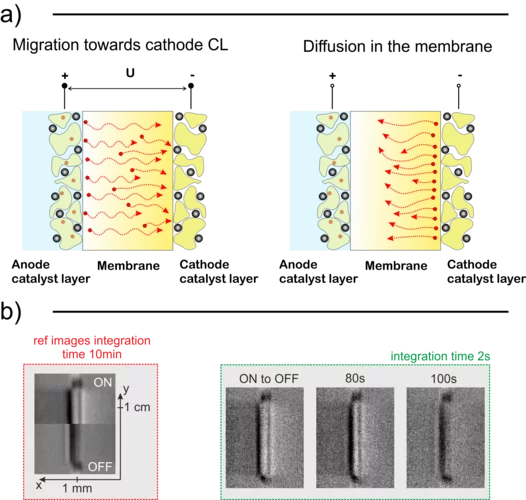
Temperature Dependent Water Transport Mechanism
Subsecond and submicron operando X-ray tomographic microscopy (XTM) was applied to reveal the water dynamics inside the gas diffusion layer (GDL) of polymer electrolyte fuel cells (PEFC). Utilizing the instrumental advancements in operando XTM of PEFCs the contribution of capillary-fingering and phase-change-induced flow on water transport in GDLs was quantified, for the first time during fuel cell startup at different operation temperatures.
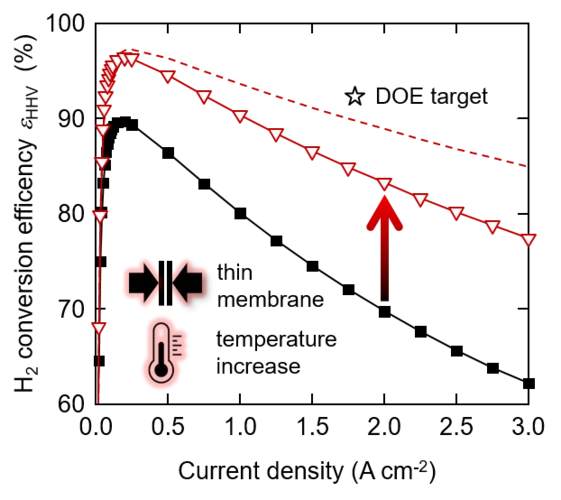
Efficient Water Electrolysis at Elevated Temperature using Commercial Cell Components
Decarbonization of the energy system across different sectors using power-to-X concepts relies heavily on the availability of low-cost hydrogen produced from renewable power by water electrolysis. Polymer electrolyte water electrolysis (PEWE) is a promising technology for hydrogen (and oxygen) production for distributed as a well as centralized operation. The total cost of hydrogen is dominated by the electricity cost. Therefore, increase of conversion efficiency is pivotal in improving the commercial viability of electrolytically produced hydrogen. In this study, we investigate the prospects of improving conversion efficiency by reducing the membrane thickness from 200 to 50 micron and increasing the cell temperature from 60 to 120°C.
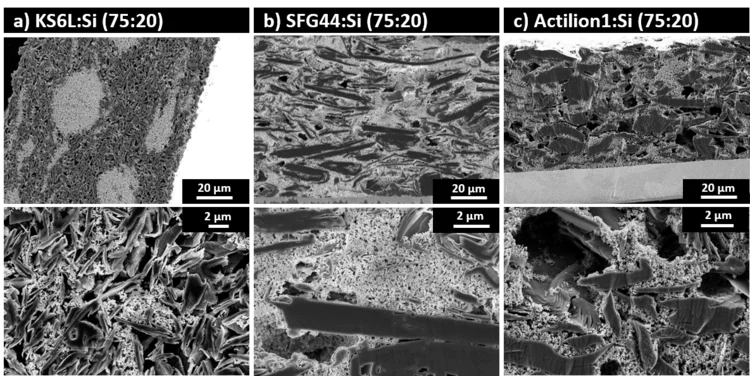
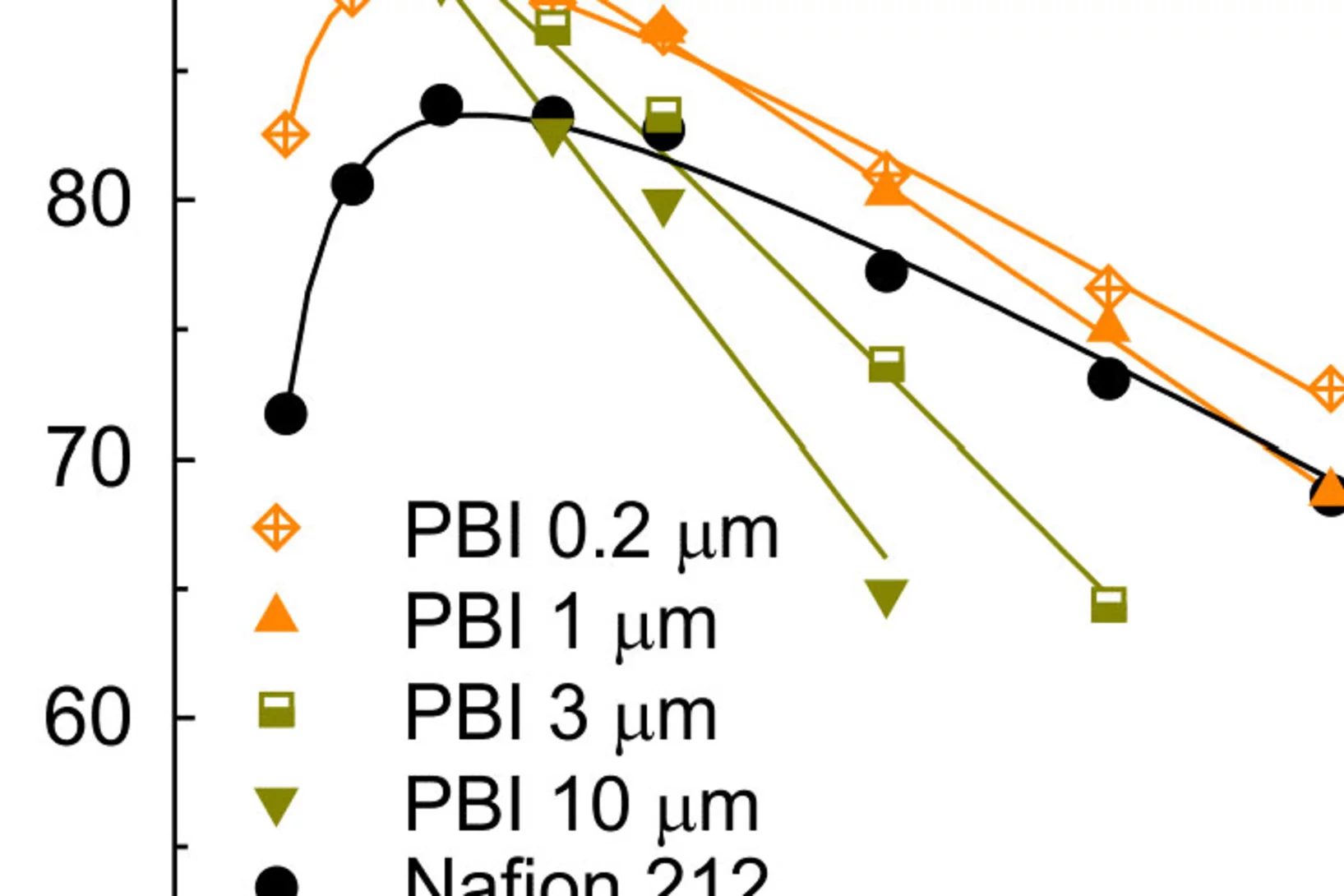
Graphite Anodes with Si as Capacity-Enhancing Electrode Additive
Silicon is a long-standing candidate for replacing graphite as the active material in negative electrodes for Li-ion batteries, due to its significantly higher specific capacity. However, Si suffers from rapid capacity loss, as a result of the large volume expansion and contraction during lithation and de-lithiation. As an alternative to pure Si electrodes, Si could be used as a capacity-enhancing additive to graphite electrodes.
LEC Contribution to the SCCER Mobility Project
Two groups from the Electrochemistry Laboratory, together with a team from ZHAW and EPFL have developed a novel evaporative cooling concept for polymer electrolyte fuel cells from the material to the cell level.
The 5 min movie explains and summarizes the development.
Oxygen Evolution Reaction Activity and Underlying Mechanism of Perovskite Electrocatalysts at Different pH
PSI researchers have studied the how the electrolyte pH values influence the oxygen evolution reaction (OER) activity and stability of different promising perovskite oxide catalysts for application as anodic electrodes in alkaline water electrolyzers. The OER activity and stability decreased decreasing the electrolyte pH values. By combining electrochemical studies and operando X-ray absorption spectroscopy measurements, it has been suggested that different reaction mechanisms dominate in alkaline and near-neutral electrolyte pH region.
Analysis of cation contamination of polymer electrolyte water electrolysers (PEWEs)
With the help of in situ PEWE regeneration methods, we can potentially enable the treatment of degraded cells without the necessity of stack disassembly, saving operation costs of the plant. In this context, we observed the movement of cations in a PEWE cell using neutron imaging and compared it with a model. This model is expected to be useful for the early detection of cation contamination problems in PEWEs, and the monitoring of in situ regeneration.
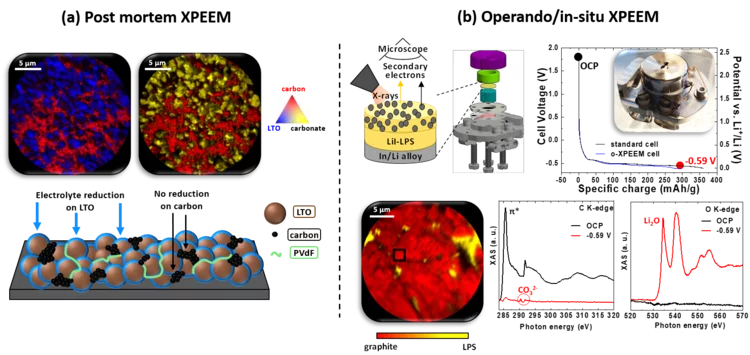
Post mortem/operando XPEEM: for studying the surface of single particle in Li-ion battery electrodes
X-ray photoemission electron microscopy (XPEEM) with its excellent spatial resolution is a well-suited technique to elucidate the complex electrode-electrolyte interface reactions in Li-ion batteries. It provides element-specific contrast images and enables the acquisition of local X-ray absorption spectra on single particles. Here we demonstrate the strength of post mortem measurements and we show the first electrochemical cell dedicated for operando experiments in all-solid-state batteries.
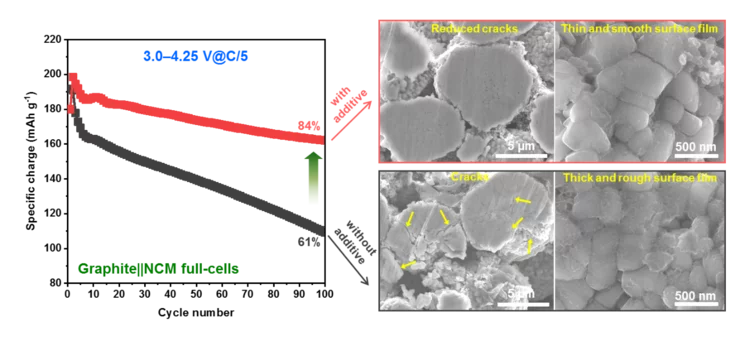
Improved Interfacial Stability of Ni-rich Oxide Full-Cells
PSI researchers have identified a novel electrolyte additive, allowing extended voltage range of Ni-rich oxide full-cells, while keeping excellent performance. The instability of cathode–electrolyte interface causes the structural degradation of cathode active material and the electrolyte consumption, resulting in a rapid capacity fading and shortening battery life-time. The PSI-identified additive help to alleviate these problems and extend battery life-time.
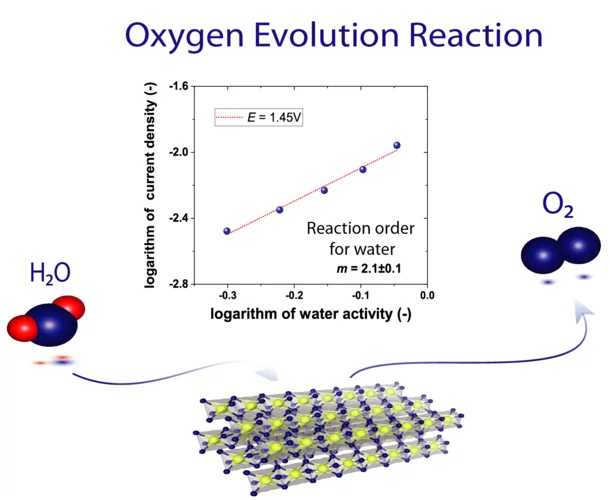
Understanding of the Oxygen Evolution Reaction Kinetics in Acidic Environment
The high operational expenditure of polymer electrolyte water electrolysis (PEWE) technology, dominated by kinetic losses from the sluggish oxygen evolution reaction (OER), inhibits large-scale market penetration. PSI researchers have developed a novel methodology to access underlying reaction mechanism of the OER. For the first time the reaction order for water has been determined. Advanced benchmarking of catalysts in technical environment also supports the development of novel, highly efficient catalyst materials.
Bilayer Composite Membrane for the Vanadium Redox Flow Battery
The vanadium redox flow battery (VRFB) is designed for grid-scale energy storage applications. Ion-exchange membranes are performance and cost relevant components of redox flow batteries. Currently used materials are largely ‘borrowed’ from other applications that have different functional requirements. For next generation VRFBs, it would be desirable to develop membrane materials based on low-cost porous separators with low resistance and high transport selectivity to minimize vanadium-ion and electrolyte crossover.
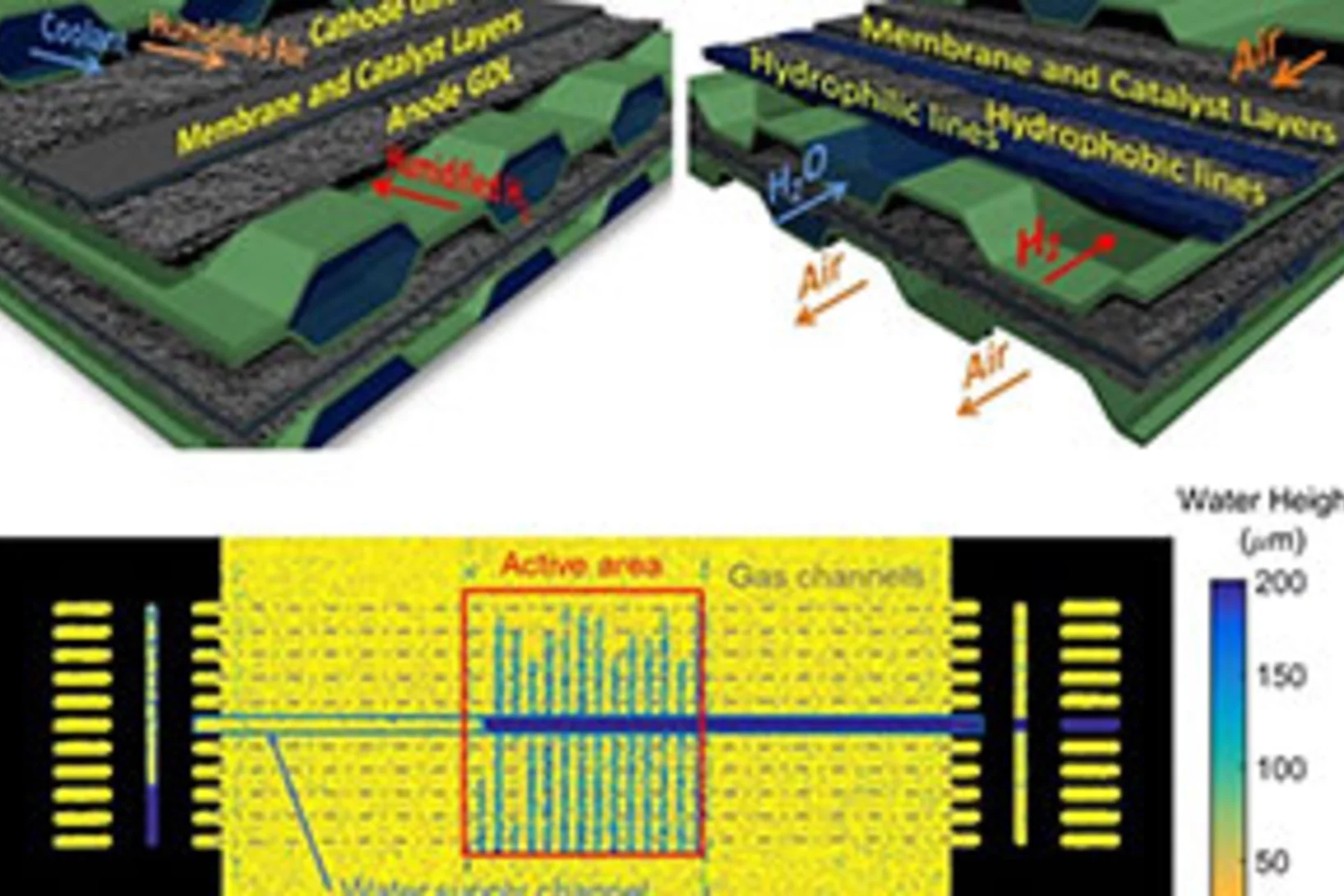
How to increase the power density of Polymer Electrolyte Fuel Cells with evaporative cooling
A system of evaporative cooling for Polymer Electrolyte Fuel Cells (PEFCs) has been developed at PSI, based entirely on one simple, yet effective change of one of the fuel cell components. Our team at the Electrochemistry Laboratory has demonstrated how this single change allows to operate a cell without the need of bulky and costly external humidifiers. Additionally, the proposed design has the potential to increase the power density of a PEFC stack by up to 35% due to the sparing of the space usually dedicated to the convective coolant circulation.
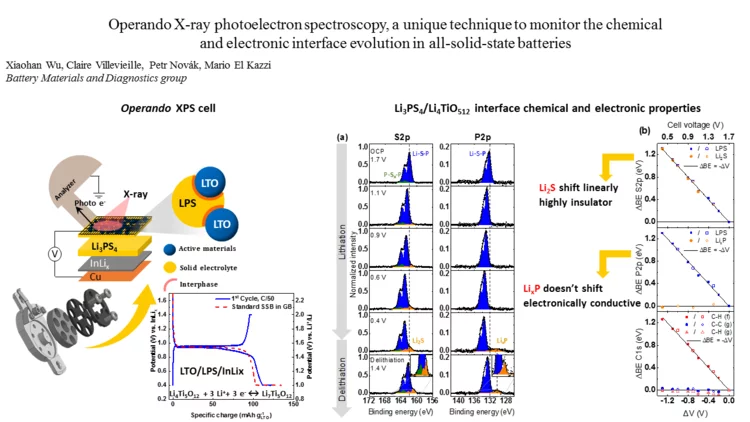
Operando X-ray photoelectron spectroscopy, to monitor the chemical and electronic interface evolution in all-solid-state batteries
Degradation of the solid-electrolyte interface occurring during cycling is currently one of the most challenging issues for the development of all-solid-state batteries. Here we designed a unique electrochemical custom made cell for operando X-ray photoelectron spectroscopy (XPS) capable of maintaining high mechanical pressure with reliable electrochemistry and able to monitor in real-time the surface (electro-)chemical reactivity at the interfaces between the different composite.
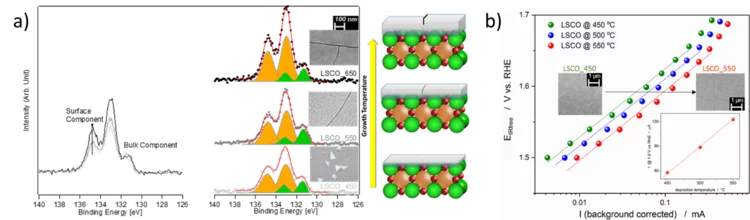
Surface segregation acts as surface engineering for the oxygen evolution reaction on perovskite oxides in alkaline media
PSI researchers have studied the influence of surface segregation on the oxygen evolution reaction (OER) activity for the, La0.2Sr0.8CoO3-d (LSCO) perovskite, one of the most active perovskite towards the OER in alkaline electrolyte. It has been found that the higher the perovskite synthesis temperature the more strontium segregation occurs on the surface. However, the segregated strontium compounds are soluble in water and they are easily removed when the surface of the electrode is in contact with the electrolyte, leading to the exposure of cobalt enriched layers very active for the OER.
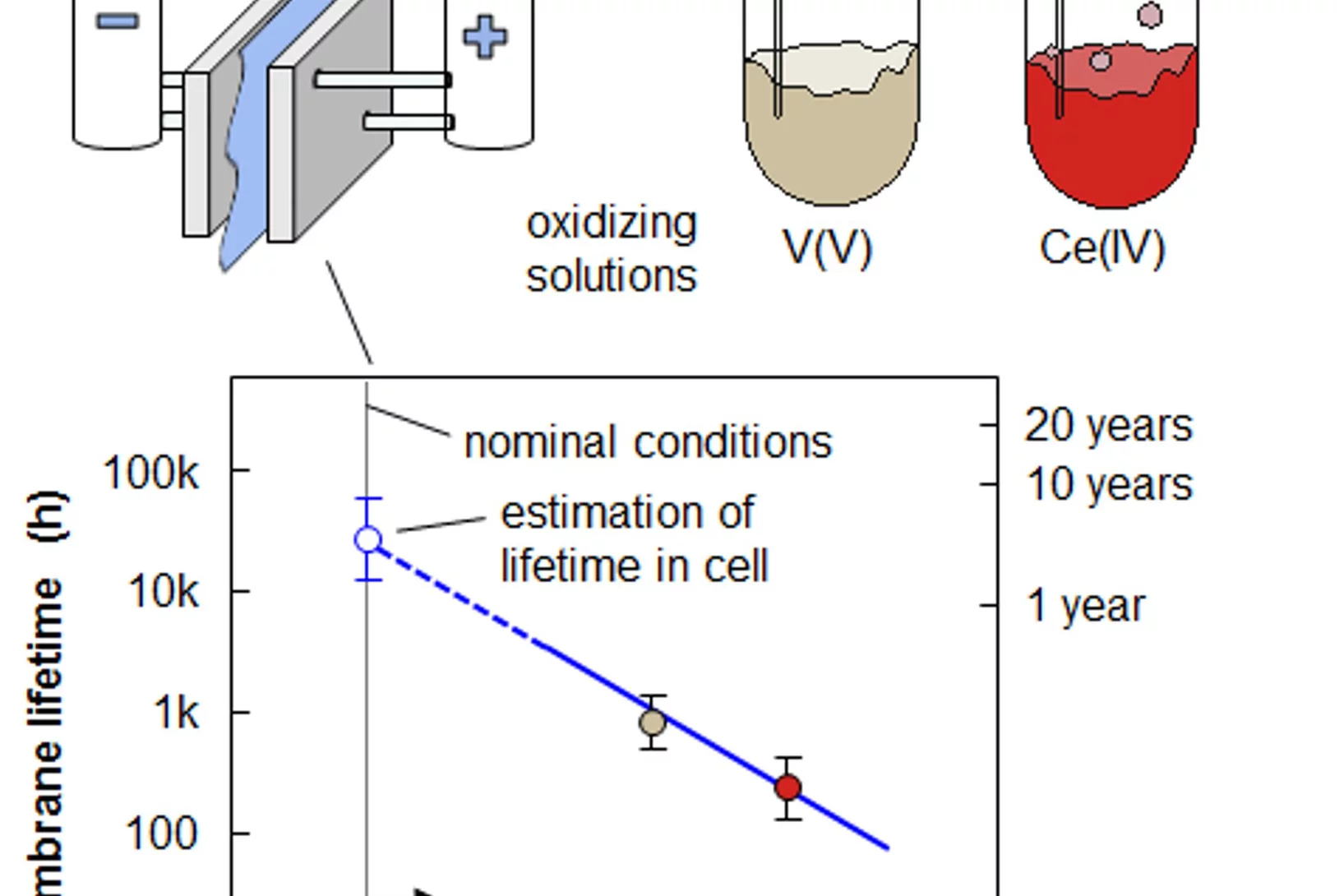
Membrane Lifetime Estimation in a Vanadium Redox Flow Battery using an Accelerated Stress Test
A vanadium redox flow battery (VRFB) is a grid-scale energy storage device. Its energy conversion unit consists of a cell stack that comprises ion-exchange membranes to separate positive and negative electrode. The projected lifetime of a VRFB is 20 years and 7’000 charge-discharge cycles. Lifetime tests of membranes under application relevant conditions are therefore impractical, and the development of an accelerated stress test (AST) to assess the chemical stability of membranes is crucial.
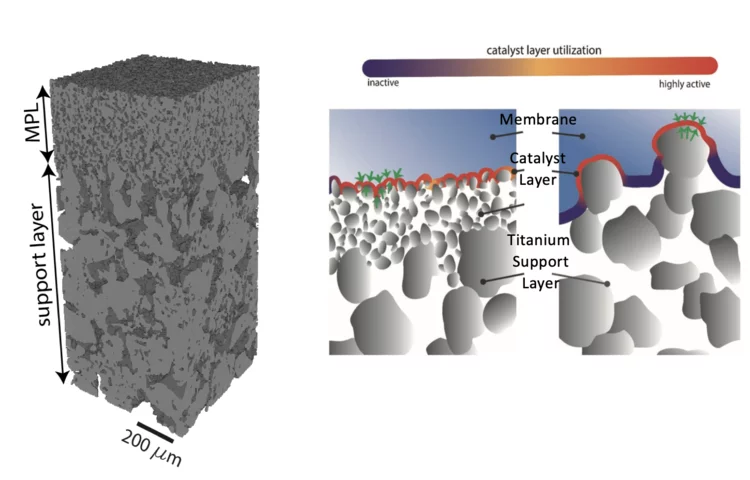
Hierarchically Structured Porous Transport Layers for Polymer Electrolyte Water Electrolysis
The high operational and capital costs of polymer electrolyte water electrolysis technology originate from limited catalyst utilization and the use of thick membrane electrolytes. PSI researchers have developed novel multi-layer porous transport materials, which provide superior electrochemical performance in comparison to conventional single-layer structures.
Vom Walkman zum Elektroauto
Drei Forscher teilen sich dieses Jahr den Nobelpreis in Chemie. Sie werden ausgezeichnet für ihren jeweiligen Beitrag zur Entwicklung von Lithium-Ionen-Batterien. Petr Novák forscht am PSI ebenfalls in diesem Bereich und kennt die drei Preisträger seit Jahrzehnten persönlich. Im Interview erzählt er, wie er einem von ihnen im entscheidenden Moment gegenübersass.
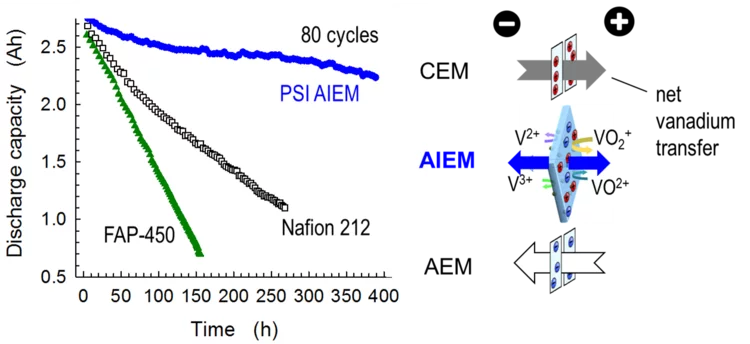
Progress Towards Designed Membranes For All-Vanadium Redox Flow Batteries
Grid-scale storage of electricity is vital in energy scenarios with a high share of renewable electricity generation, such as wind and solar power. Redox flow batteries are particularly suited for intra-day time-shifting storage applications, yet investment costs need to be lowered for economic viability of the technology. We demonstrate a new ion conducting membrane that improves shortcomings of currently used materials and is potentially cheaper to produce.
Brennstoffzellen zum Durchbruch verhelfen
Wasserstoff gilt als vielversprechende Alternative für eine Zukunft ohne fossile Energieträger. Um Brennstoffzellen weiterzuentwickeln und für einen Markteintritt vorzubereiten, verstärkt die Empa die Zusammenarbeit mit der H2 Energy Holding AG und dem Paul Scherrer Institut (PSI).