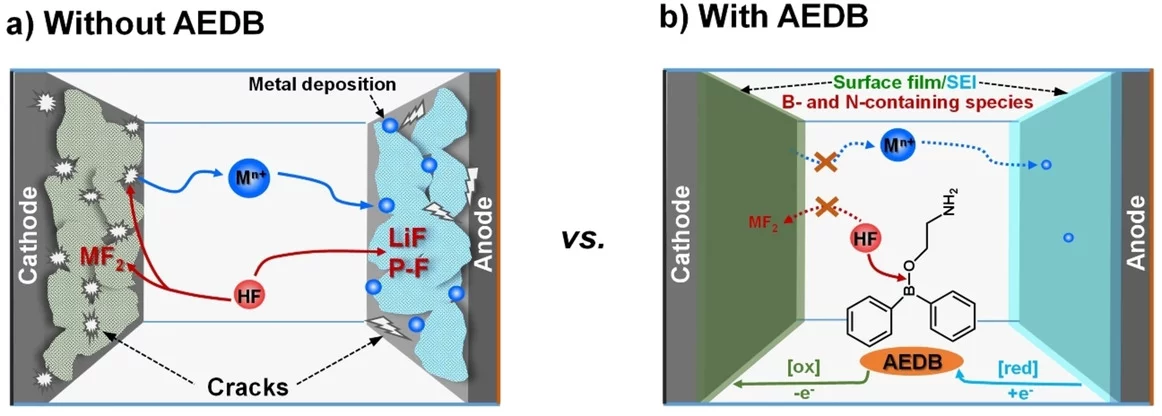
Control of interfacial reactivity at high-voltage is a key to high-energy-density Li-ion batteries. 2-aminoethyldiphenyl borate was investigated as an electrolyte additive to stabilize surface and bulk of both NCM851005 and graphite in the cell with upper cut-off voltage of 4.4 V vs Li+/Li. AEDB almost completely eliminated the “cross-talk” in the cell, by significantly reducing metal leaching from the cathode, preventing their deposition at the anode, and further electrolyte decomposition.
Energy density of Li-ion cells can be increased by using materials with higher capacity, extending operating voltage window, or both. The classical cathode active materials, based on intercalation chemistry have limited capacity of below 300 mAh/g, most often even below 200 mAh/g. Therefore, here the strategy is to increase battery cell’s upper cut-off potential, resulting not only in higher voltage of the cell but also extracting additional capacity from the cathode. The hurdles in following this strategy often are related to electrolyte instability at high potentials and absence of stable interface formation at the cathode surface. Control of electrode-electrolyte interfacial reactivity at high-voltage, therefore, is a key to successfully obtain high-energy-density lithium-ion batteries. In this study, 2-aminoethyldiphenyl borate (AEDB) was investigated as a multifunctional electrolyte additive in stabilizing surface and bulk of both Ni-rich LiNi0.85Co0.1Mn0.05O2 (NCM851005) and graphite electrodes with elevated upper cut-off voltage of 4.4 V vs Li+/Li. The AEDB improved performance of both cathode and anode materials in a full-cells, suppressing crack formation of cathode particles and helping to preserve the structure of oxide and graphite after multiple cycles. Moreover, even if the effect of AEDB in half-cells was rather modest, in full-cells its addition resulted in tremendous performance improvement. The graphite‖NCM851005 full-cell in the presence of AEDB has a capacity retention of 88 % after 100 cycles, even when the upper cut-off voltage was 4.35 V, corresponding to 4.4 V vs Li+/Li, whereas with standard electrolyte under the same conditions the retention was only 21 %. Most importantly, AEDB almost completely eliminated so called “cross-talk” between the two electrodes, by significantly reducing metal dissolution from the cathode, preventing them being deposited at the anode, where they could catalyse further electrolyte decomposition. Noteworthy is the fact that the effect of the AEDB in half-cells is rather unimpressive, while in the full-cell it shows the full potential, showing the importance of additive testing to be performed in the environment of their intended use - the full-cell.
Contact
Dr. Sigita Trabesinger
Group Head Battery Electrodes and Cells
Paul Scherrer Institut
5232 Villigen PSI
Switzerland
Telephone: +41 56 310 57 75
E-mail: sigita.trabesinger@psi.ch
Original Publication
Cross-Talk-Suppressing Electrolyte Additive Enabling High Voltage Performance of Ni-Rich Layered Oxides in Li-Ion Batteries
Hieu Quang Pham, Minh Tri Nguyen, Mohamed Tarik, Mario El Kazzi, Sigita Trabesinger
ChemSusChem 14, 1–15 (2021)
DOI: 10.1002/cssc.202100511
Acknowledgement
This work was financially supported by the BASF Scientific Network on Electrochemistry and Batteries, and the SCCER Heat and Electricity Storage.