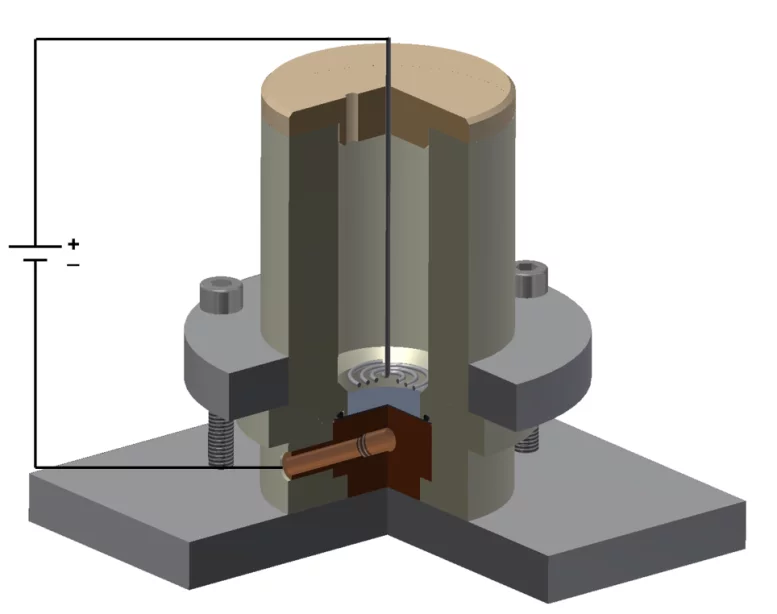
Molecular plating is one of the most used techniques for target preparation, first introduced in 1962 by Parker et al. [1]. This technique consists in electrodepositing compounds from an organic solvent. It is generally performed in a cell containing two electrodes, where the cations are electrodeposited onto the working electrode, i.e. the cathode (see Figure). The use of organic solvents allows minimizing the current density during the deposition, in the order of a few milliamps per cm2, thus minimizing the rate of H2 production in the cathode, which may interfere with the layer formation. On the other hand, the low current does not allow the cations to be reduced to their metallic form. As a matter of fact, the chemical process involved in the deposition of elements and their final chemical speciation are still not well known. The advantages of this technique are: high deposition yields (85-95%), obtainment of uniform and homogeneous targets. The drawback is that the deposited layer is usually relatively thick, in the order of some hundreds of µg/cm2 (some µm).
[1] W. Parker, R. Falk, Molecular plating: A method for the electrolytic formation of thin inorganic films, Nucl. Instr. Meth., 16 (1962) 355.