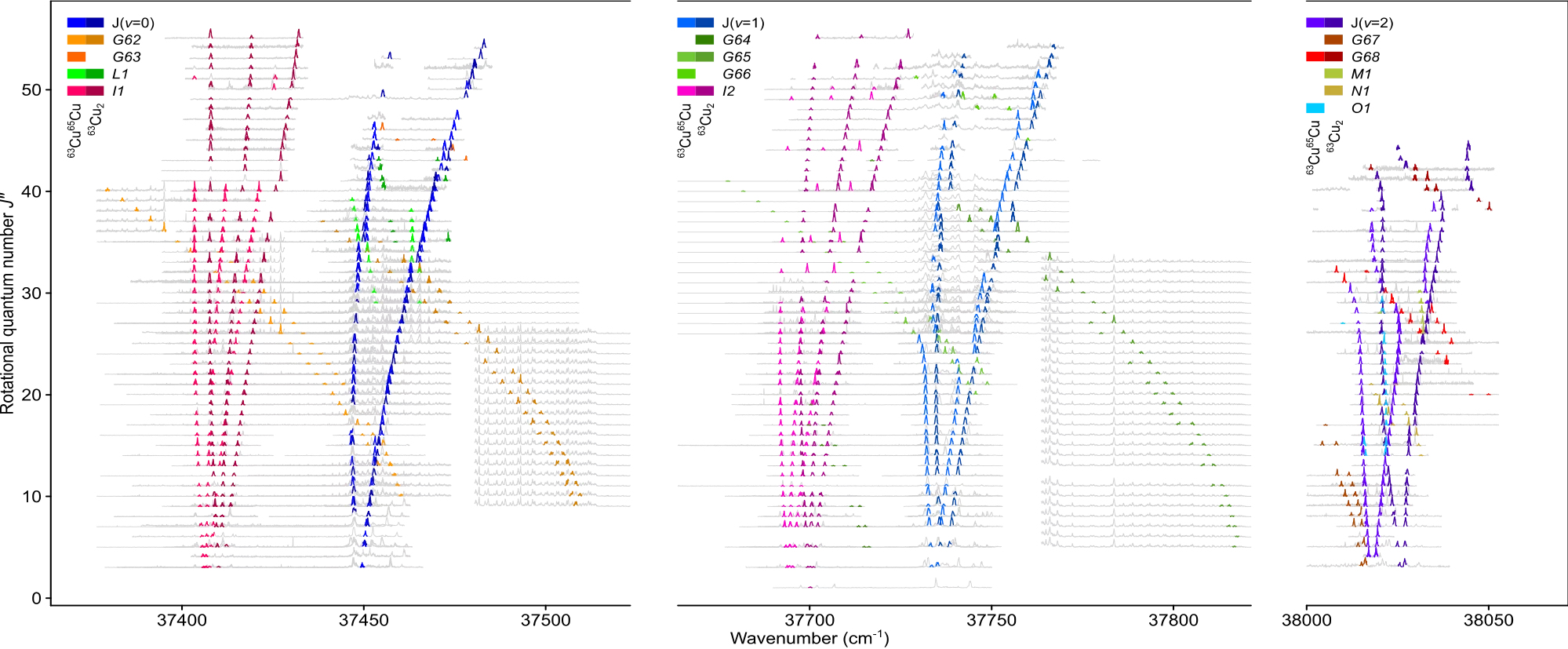
Transition metals, characterized by their partially filled d-orbitals, provide the basis for many of the most relevant processes in chemistry, biology, and physics. Embedded as single atoms or in small clusters, they give rise to exceptional optical, chemical, and magnetic properties. So far, it has proven impossible to disentangle the complex network of excited quantum states, which greatly hinders predicting and controlling of material properties. We employed double-resonant four-wave mixing spectroscopy to quantitatively resolve the bright and perturbing dark quantum states of the neutral copper dimer.
For the investigation, we combined a newly developed metal cluster source with a non-linear, double-resonant four-wave mixing experiment. The cluster source was optimized for the generation of a molecular beam containing a high number density of the copper dimer. With the setup, we could employ selective spectroscopy at rotational resolution to investigate the complex energy map of a transition metal dimer.
The low energy structure of the copper dimer is often considered to be the simplest of the transition metal dimers, with just two molecular states arising from the combination of ground state 2S (3d104s) atoms. The molecular ground state of the molecule, X1Σu+(0u+) and the first excited state, a3Σu+(1u) are formed by the two electrons in the (4sσ)2 and (4sσ)(4sσ*) molecular orbitals, respectively, and there is little interaction between the filled 3d shells localized on the atoms. However, the promotion of an electron from the 3d shell leads to a much larger number of possible combinations with 40 molecular states from the bonding of a ground state atom 2S with an excited 2D atom. The combination of two 2D atoms gives already rise to 100 states, which populate the energy range up to 5 eV. The spectroscopic characterization of such a high density of molecular states is a challenging endeavor.
In this work, by exciting the copper dimer using deep-ultraviolet light (~4.5 eV) , an energy region of dicopper is investigated where it behaves like a typical transition metal possessing open-shell d-electrons: Interactions of a large number of close lying states go beyond the reach of any computation and experimental spectra are generally too complicated to be assigned. The highly selective and sensitive method applied here quantitatively unravels a rich network of interacting states. Hence, the complex bonding features involving ion-pair states, perturbations, and avoided crossings are becoming accessible. Such knowledge sheds light on the electronic structure that governs the functionality of transition metals and provides reference data for advanced computations of these important molecules. The report of this investigation was recently published in Nature Communications.[1]
The expertise on four-wave mixing techniques in the optical and VUV regime is being transferred towards new frontiers in the X-ray regime. The feasibility of creating a transient grating, an important prerequisite for non-linear four-wave mixing spectroscopy, has been demonstrated recently at the Swiss Free Electron Laser facility.
[1] M. Beck, P. Bornhauser, B. Visser, G. Knopp, J. A. van Bokhoven & P. P. Radi, Nature Communications, 10, 3270 (2019). https://doi.org/10.1038/s41467-019-11156-2