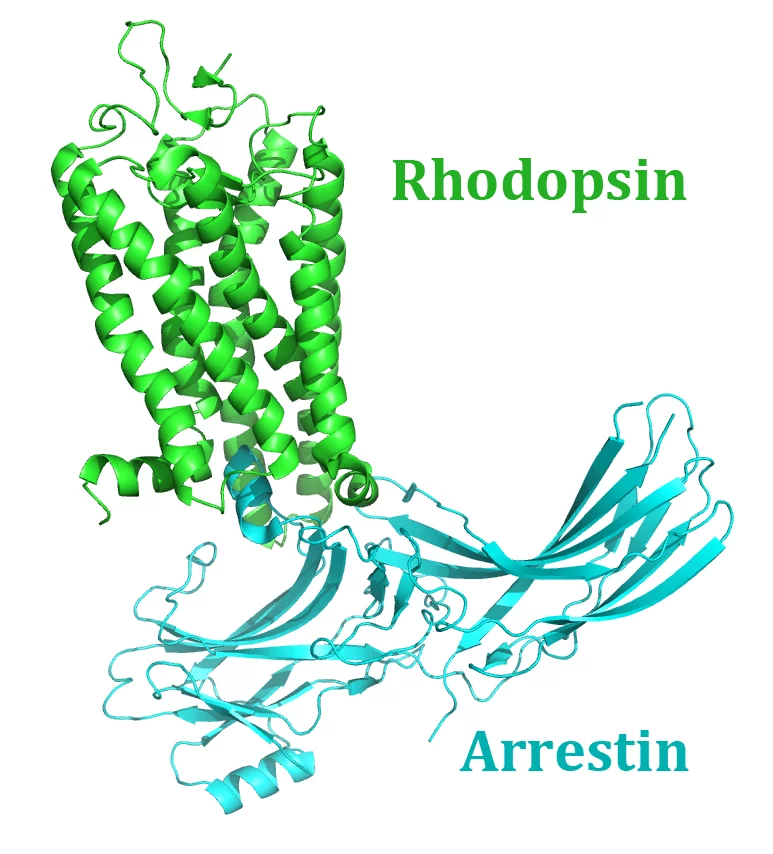
A team of 72 investigators across 25 institutions including researchers from the Paul Scherrer Institut obtained the X-ray structure of a rhodopsin–arrestin complex, which represents a major milestone in the area of G-protein-coupled-receptor (GPCR), a protein family recognized in the award of the 2012 Nobel Prize in Chemistry.
The study, “Crystal Structure of Rhodopsin Bound to Arrestin Determined by Femtosecond X-ray Laser”, which was published in the journal Nature, provides insight into the interactions between G-protein-coupled-receptors (GPCRs) and arrestins, proteins that regulate vital physiological functions such as sensory and hormonal responses. The GPCR rhodopsin is involved in one of the best-characterized GPCR signalling pathway that controls the process of visual phototransduction in the retina of the eye.
Many challenges were faced in the process of solving the X-ray structure of the rhodopsin–arrestin complex, in particular the very small and weakly diffracting crystals proved unsuitable for conventional synchrotron experiments. Only the ultrabright X-rays from the free-electron laser (FEL) at the SLAC National Accelerator Laboratory (Menlo Park, California) enabled the research team to obtain the structure at the atomic scale.
This finding represents an important step in designing new treatment against the many diseases that are mediated by GPCR signalling pathways, which are the target for almost half of the drugs currently on the market.
Such high-impact scientific discoveries will soon be possible at the new large-scale facility, SwissFEL, currently being built at the Paul Scherrer Institut.
Many challenges were faced in the process of solving the X-ray structure of the rhodopsin–arrestin complex, in particular the very small and weakly diffracting crystals proved unsuitable for conventional synchrotron experiments. Only the ultrabright X-rays from the free-electron laser (FEL) at the SLAC National Accelerator Laboratory (Menlo Park, California) enabled the research team to obtain the structure at the atomic scale.
This finding represents an important step in designing new treatment against the many diseases that are mediated by GPCR signalling pathways, which are the target for almost half of the drugs currently on the market.
Such high-impact scientific discoveries will soon be possible at the new large-scale facility, SwissFEL, currently being built at the Paul Scherrer Institut.
Reference
Quantitative interior x-ray nanotomography by a hybrid imaging techniqueYanyong Kang, X. Edward Zhou, Xiang Gao, Yuanzheng He, Wei Liu, Andrii Ishchenko, Anton Barty, Thomas A. White, Oleksandr Yefanov, Gye Won Han, Qingping Xu, Parker W. de Waal, Jiyuan Ke, M. H. Eileen Tan, Chenghai Zhang, Arne Moeller, Graham M. West, Bruce D. Pascal, Ned Van Eps, Lydia N. Caro, Sergey A. Vishnivetskiy, Regina J. Lee, Kelly M. Suino-Powell, Xin Gu, Kuntal Pal, Jinming Ma, Xiaoyong Zhi, Sebastien Boutet, Garth J. Williams, Marc Messerschmidt, Cornelius Gati, Nadia A. Zatsepin, Dingjie Wang, Daniel James, Shibom Basu, Shatabdi Roy-Chowdhury, Chelsie E. Conrad, Jesse Coe, Haiguang Liu, Stella Lisova, Christopher Kupitz, Ingo Grotjohann, Raimund Fromme, Yi Jiang, Minjia Tan, Huaiyu Yang, Jun Li, Meitian Wang, Zhong Zheng, Dianfan Li, Nicole Howe, Yingming Zhao, Jorg Standfuss, Kay Diederichs, Yuhui Dong, Clinton S. Potter, Bridget Carragher, Martin Caffrey, Hualiang Jiang, Henry N. Chapman, John C. H. Spence, Petra Fromme, Uwe Weierstall, Oliver P. Ernst, Vsevolod Katritch, Vsevolod V. Gurevich, Patrick R. Griffin, Wayne L. Hubbell, Raymond C. Stevens, Vadim Cherezov, Karsten Melcher and H. Eric Xu
Nature 2015; 523 (7562):561-7 DOI:10.1038/nature14656
More information about Switzerland’s free-electron laser
Current News SwissFEL
Homepage of the SwissFEL project
Contact
Dr. Meitian Wang, Swiss Light SourcePaul Scherrer Institut, 5232 Villigen PSI, Switzerland
Phone: +41 56 310 4175, e-mail: meitian.wang@psi.ch
Dr. Joerg Standfuss, Swiss Light Source
Paul Scherrer Institut, 5232 Villigen PSI, Switzerland
Phone: +41 56 310 2586, e-mail: joerg.standfuss@psi.ch