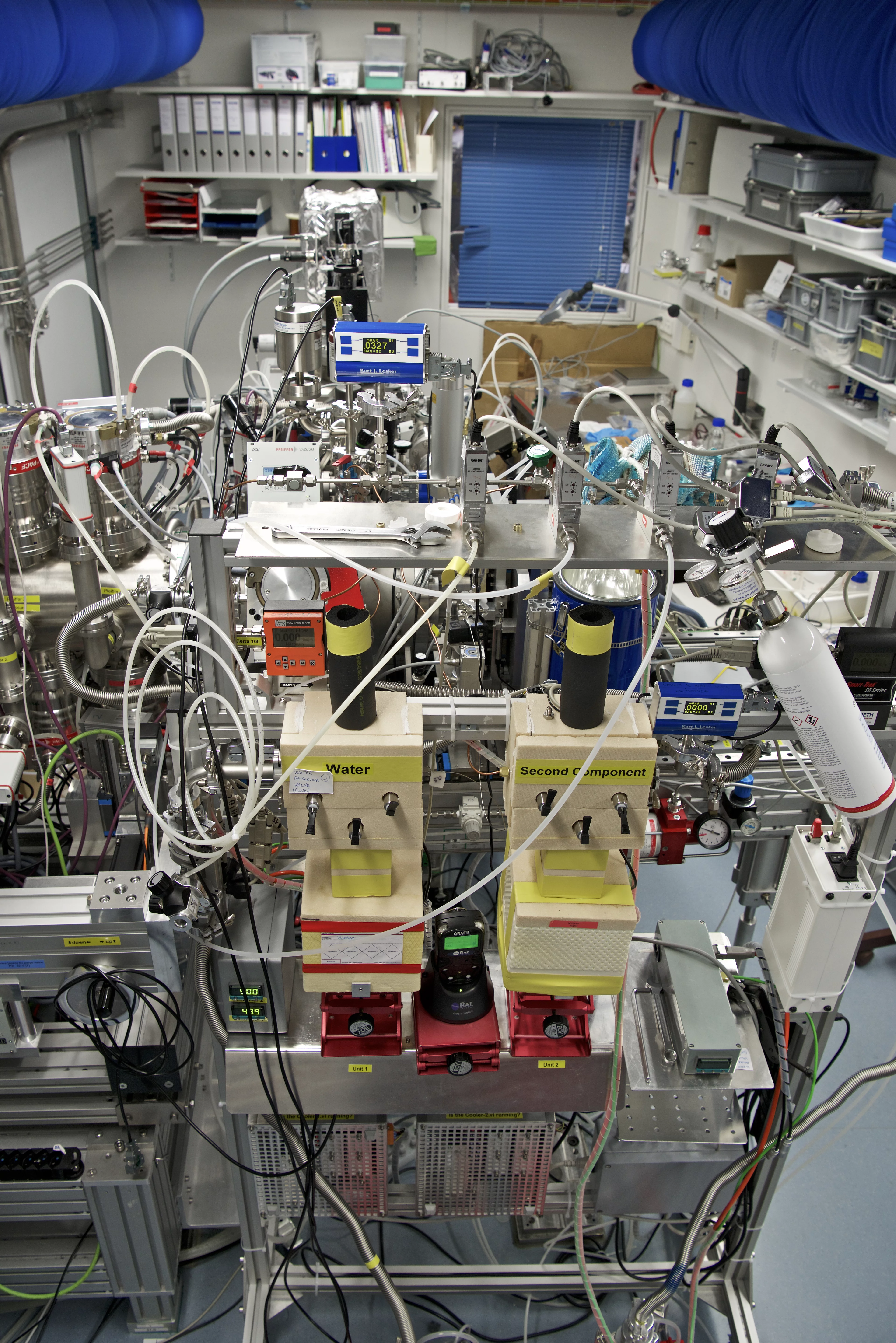
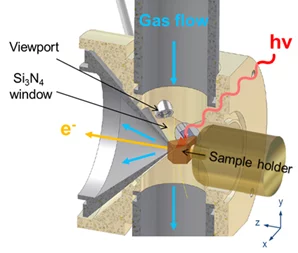
Such a configuration mimics a flow tube reactor so that both environmental chemistry/surface chemistry and heterogeneous catalysis experiments can be carried out. The cell can operate in static mode (pumped only from the aperture of the analyzer) or in flow configuration. The maximum pressure while measuring depends on the excitation energy used. With soft x-rays, photoemission spectra can be acquired up to 5 mbar. Sample treatments are possible up to 50 mbar. The pressure is measured by means of Baratron capacitance heads integrated in a Labview interface.
The small volume of the experimental cell and the flow tube configuration allow fast gas switching from one mixture to another (in the second timescale), while acquiring photoemission spectra in fast-scan mode. We were recently introducing time-resolved measurements, and could get information about the evolution of real samples (powder catalyst) in a specific reaction mixture as a function of time in the second range [2]. Time resolution is one of the next frontiers in in-situ XPS and will make possible to follow dynamic structural changes that typically occur in catalysts. It will also provide quantitative structure-performance relations previously not accessible.
Manipulators, Samples, Topics
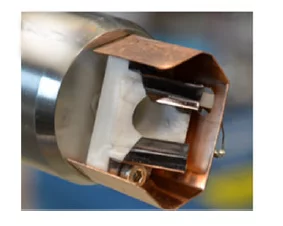
Heated sample holder
The sample is heated by means of a IR laser (976 nm, maximum power=25 W) projected on the back side of the sample holder. The highest temperature achieved strongly depends on the gas used and/or on the pressure in the experimental cell. For most of the experimental conditions, it is possible to work between (25oC) and 650oC. The temperature is measured by means of a Pt100 temperature sensor, and is set with a PID controller integrated in a Labview interface.
Both flat samples (e.g. foils and single crystals) and powders can be mounted and investigated. Maximum dimension of flat samples is 10x10 mm. Powder samples can be either suspended in an appropriate solvent and drop-casted on a support foil or pressed into pellets (press die available).
This manipulator insert, combined with the unique features of the flow reaction cell, is suited for surface chemistry and catalysis experiments.
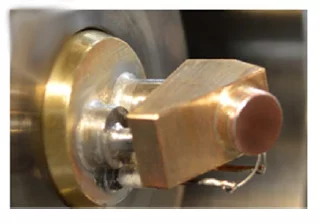
Cryo holder
This manipulator has a cooling circuit insulated from air by means of a differential pumping stage. A cold finger creates the contact between the cooling circuit and the sample holder surface. Such a design ensures that only the sample holder/sample surface is cooled, while the rest of the manipulator hear is at room temperature. The temperature, monitored by means f a Pt100 sensor and set by a PID controller integrated in a Labview interface, can be finely adjusted (+-0.1oC) between -100oC and 100oC. For example, this is extremely important to tune the relative humidity inside the cell (setting a precise water pressure and the sample temperature accordingly) and/or to grow equilibrium ice [3,4].
Equilibrium ice can be grown directly on the sample holder, dosing a stable water vapor pressure in the cell and tuning the temperature to reach the saturation point. Powder and flat samples (e.g. foils and single crystals) can be drop-casted and mounted, respectively, on the sample holder.
This manipulator insert, combined with the unique features of the flow reaction cell, is perfectly suited for surface chemistry and environmental chemistry experiments.
References
[1] F. Orlando, et al. Top. Catal. 2016, 59, 591-604.[2] L. Artiglia, et al. J. Phys. Chem. Lett. 2017, 8, 102-108.
[3] X. Kong, et al. J. Phys. Chem. Lett. 2017, 8, 4757-4762.
[4] T. Bartels-Rausch, et al. ACS Earth Space Chem. 2017, 1, 572-579.