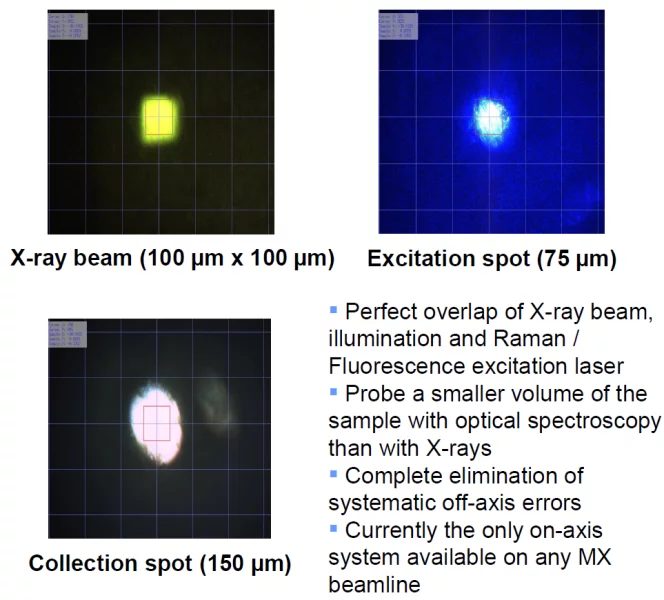
The SLS SpectroLAB facility is focused on providing the possibility for single crystal microspectrophotometry in combination with X-ray diffraction. Microspectroscopic investigation of protein crystals yields additional information, such as the redox state of metal centers or S-S bonds, identity of bound ligands, or degrees of radiation damage. The SLSpectroLAB is a general user facility at beamline X10SA (PXII) of the SLS providing the possibility of on-line and off-line multi-mode microspectrophotometry to the user community. It is currently the only facility providing on-axis geometry for all modes and thus is able to provide spectroscopic data without artifacts from probing different sample volumes (for details see below).
User Operation
External users can apply for spectroscopy measurement time at beamline X10SA through a collaboration with PSI MX scientific staff
- Please check the Spectroscopy Local Contact pages for our support and collaboration details
General Information
On-Axis Geometry
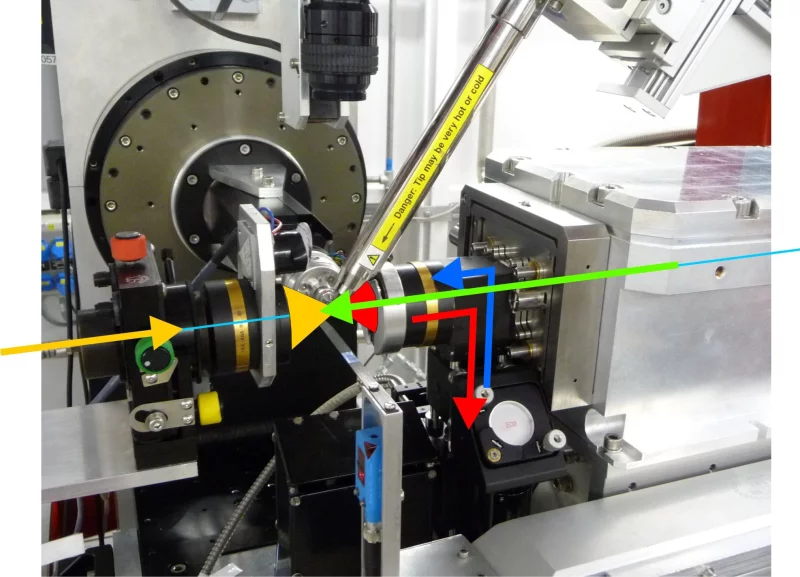
Currently the SLSpectroLAB is the only laboratory employing an on-axis geometry - co-axial alignment of imaging, spectroscopic axis and X-ray beam. In contrast to the common off-axis geometry this is an invaluable advantage when it comes to conciliating spectroscopically probed volumes of a protein crystal with the volume exposed to the synchrotron radiation (see Figure 6 below). Off-Axis configurations are extremely sensitive to probing unexposed sample volumes thus yielding compromised information. The on-axis approach required in-house construction of the imaging, excitation and collection optics.
Spectroscopic Modes
Raman Spectroscopy
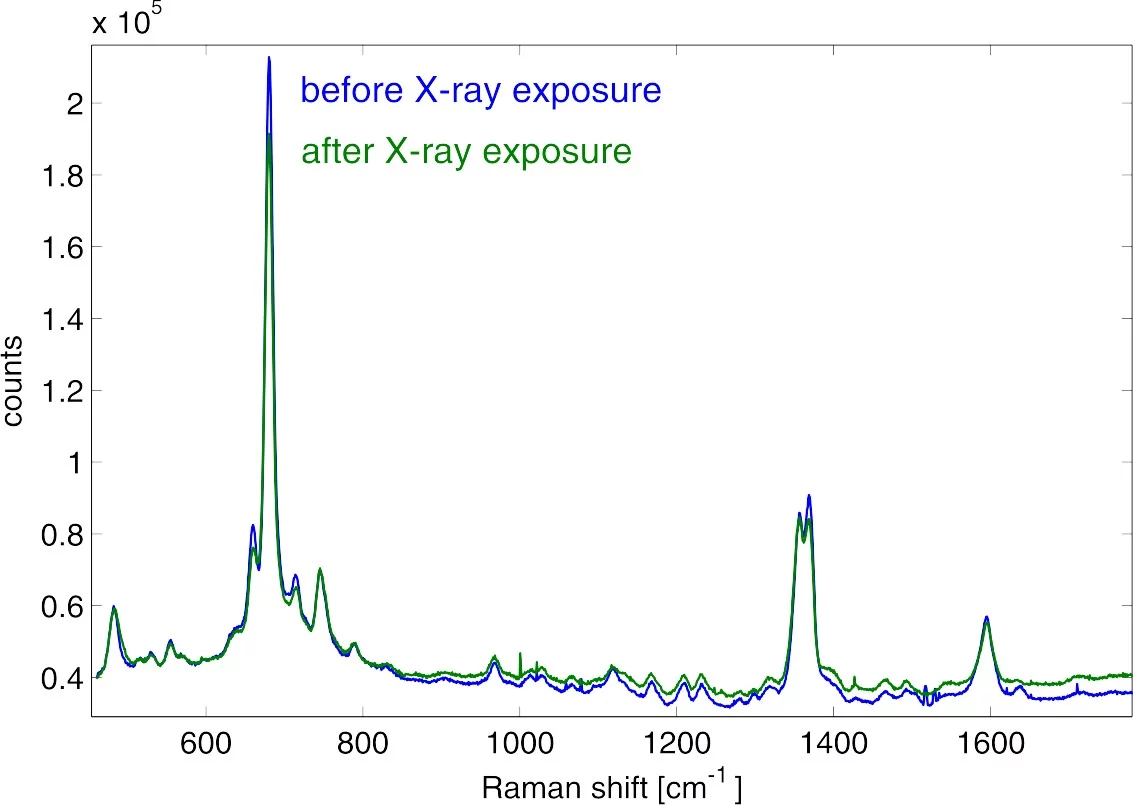
Raman spectroscopy is particularly suited for the identification of ligands and chemical species, e.g. by assignment of specific vibrational bands by difference Raman spectroscopy. A typical use case is the determination of ligand - active site interactions in soaking experiments.Resonance Raman Spectroscopy
Resonance Raman spectroscopy builds on the enhanced Raman transition probability when the excitation wavelength is approaching resonance or is in resonance with an optical transition. Apart from a general increase in signal strength, the different selectivity to the involved vibrational modes enables thr assignment of vibrational bands related to the chromophore and its direct environment.UV/Vis Absorption Spectroscopy
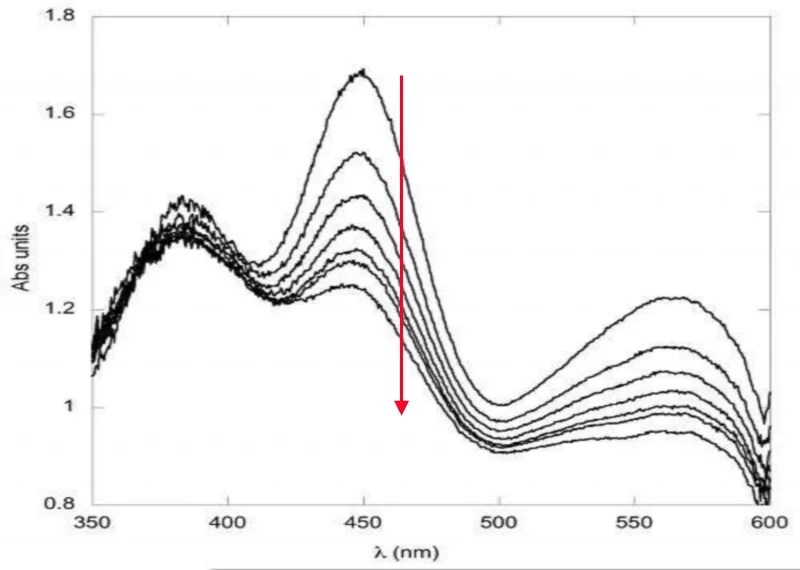
UV/Vis absorption spectroscopy is the method of choice for the observation of colored co-factors, metalloproteins and photo-sensitive proteins. In the context of X-ray diffraction experiments, a central point is the detection of radiation-induced changes such as e.g. the radiolytic reduction of metal centers or specific radiation damage such as the breakage of disulfide bridges. Of particular use in the context of structural enzymology is the possibility to characterize and monitor trapped catalytic intermediates in enzymatic reactions, both in time-resolved and static measurements.Fluorescence Spectroscopy
Fluorescence spectroscopy finally can indicate the presence of co-factors or substrates and yield information on the redox state of metal centers. It further opens the possibility to investigate the fluorescence properties of photochromic fluorescent proteins.Facility
SLS Microspectrophotometer V2
A multi-mode in-situ micro-spectrophotometer (MS) has been developed by the MX group at the SLS to perform UV/Visible absorption, fluorescence and Raman spectroscopy on protein crystals. The on-axis geometry ensures an optimal overlap between the X-ray beam and the spectroscopic probes, e.g. for a better characterization of specific radiation damage and more generally photo-induced reactions due to X-ray exposure.Off-Line Facility
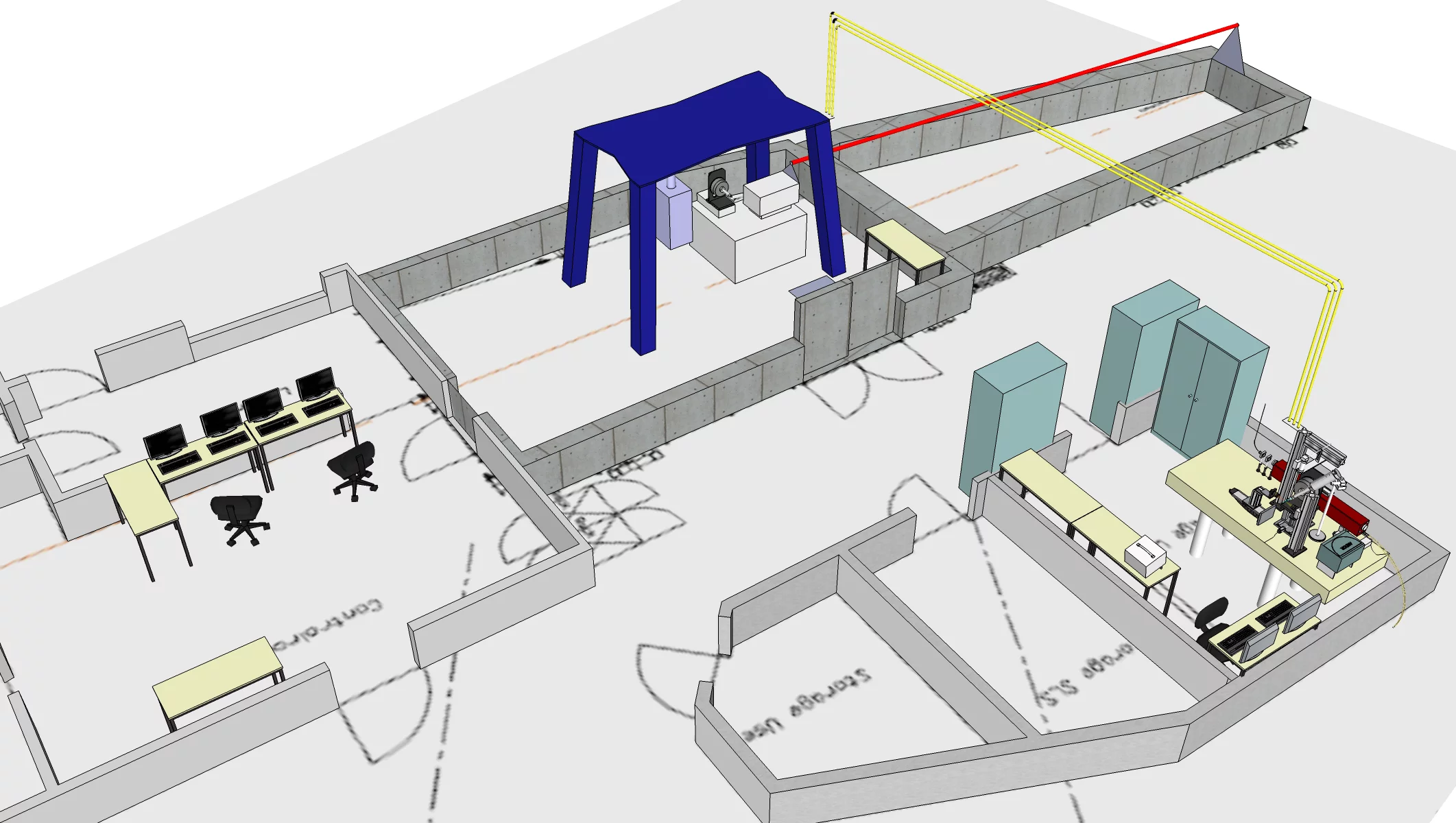
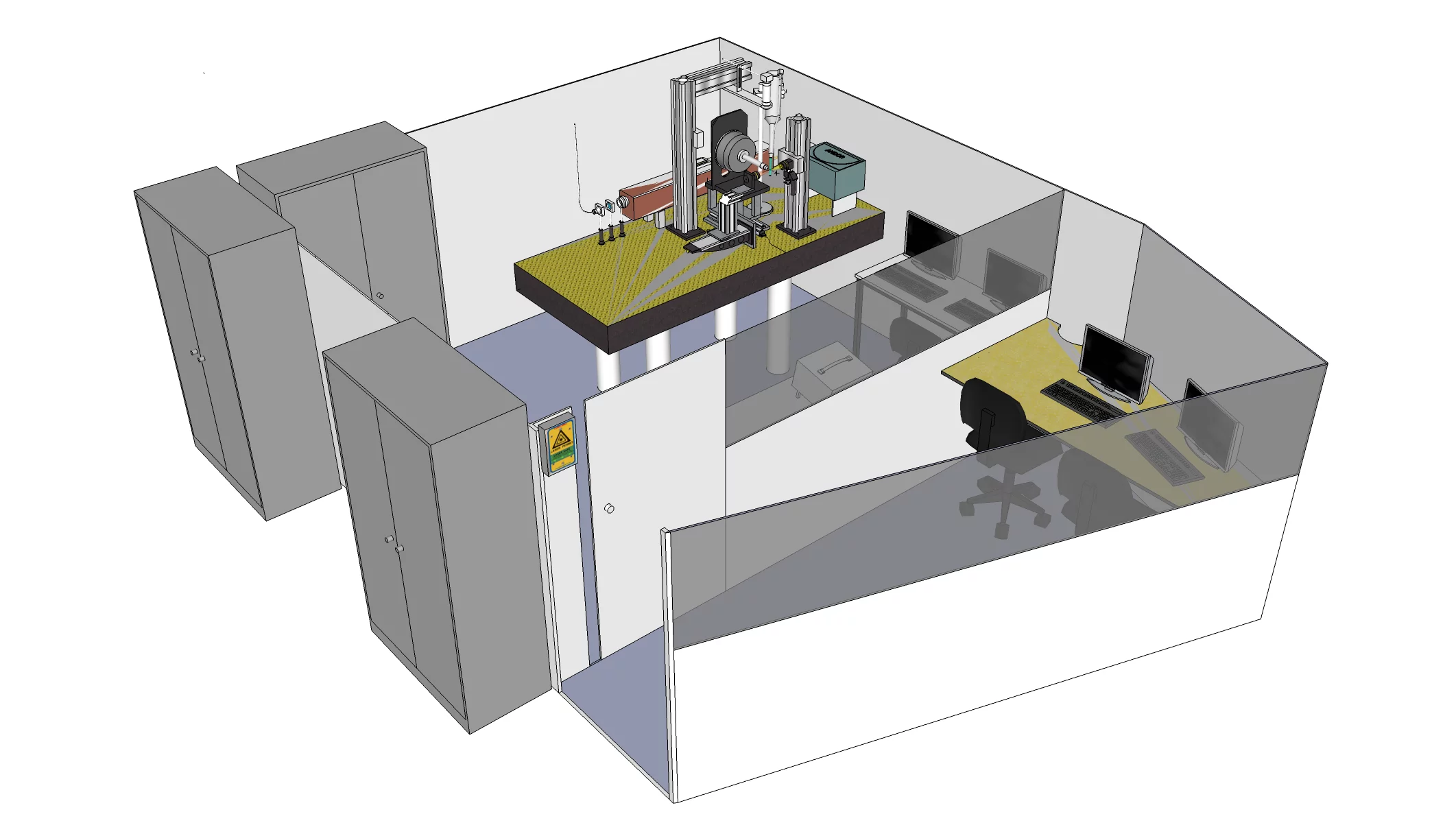
The facility consists of two hutches. The first is housing the control room with the terminal PCs. From here one can access the actual experimental hutch which is interlocked during the experiment according to the laser safety regulations. The experimental hutch houses a full crystallographic goniometer setup similar to the one used at the PXII beamline to offer all possibilities of the on-line setup. Just as at the beamline , a Cryojet cryo-cooling device is available for cooling the samples during the experiment.
Equipment
LASER
Various single-wavelength diode laser are available as well as a Coherent Innova302C Kr$^+$ Ion Laser. This allows for a multitude of different systems to be measured at the SLSpectroLAB.| Available Laser at the SLSpectroLAB | ||||
|---|---|---|---|---|
| Wavelength | Laser Type | Application | Power | Status |
| 266nm | SWD | UV illumination | xxx mW | OK |
| 405nm | SWD | RRaman | 350 mW | OK |
| 413nm | Kr | RRaman | 200 mW | CUA |
| 473nm | SWD | RRaman | 150 mW | OK |
| 532nm | SWD | Raman/Photo-bleaching | 200 mW | OK |
| 594nm | SWD | RRaman | 250 mW | OK |
| 647nm | Kr | Raman, RRaman | >300 mW | CUA |
| 785nm | SWD | Raman | >500 mW | OK |
| OK: Available ° CUA: Currently unavailable ° N/A: Not available | ||||
Other Lightsources
For UV/Vis absorption spectroscopy we utilize a ZEISS Xenon-Arc lamp with sufficient power to illuminate even larger crystals. For higher light intensity in the deep UV, a combined Deuterium/Halogen fiber-coupled light source is provided.References
How to Cite SLSpectroLAB
If you publish results you gained at the SLSpectroLAB or otherwise want to acknowledge the SLSpectroLAB, please use the following reference(s):- Pompidor, G., F.S.N. Dworkowski, et al. (2013). "A new on-axis micro-spectrophotometer for combining Raman, fluorescence and UV/Vis absorption spectroscopy with macromolecular crystallography at the Swiss Light Source." Journal of Synchrotron Radiation 20(5): 765-776. DOI:10.1107/S0909049513016063
Selected Publications
2018
- Horrell, S., D. Kekilli, et al. (2018). "Enzyme catalysis captured using multiple structures from one crystal at varying temperatures" IUCrJ 5(3): 283-292 DOI:10.1107/S205225251800386X [UVVis]
2017
- Kekilli, D., T. Moreno-Chicano, et al. (2017). "Photoreduction and validation of haem–ligand intermediate states in protein crystals by in situ single-crystal spectroscopy and diffraction" IUCrJ 4(3): 263-270 DOI:10.1107/S2052252517002159 [RRAMAN/UVVis]
- Kekilli, D., C.A. Petersen, et al. (2017). "Engineering proximal vs. distal heme–NO coordination via dinitrosyl dynamics: implications for NO sensor design." Chem. Sci. 8(3): 1986-1994 DOI:10.1039/C6SC04190F [RRAMAN]
2015
- Ghafoor, D.D., D. Kekilli, et al. (2015). "Hydrogen bonding of the dissociated histidine ligand is not required for formation of a proximal NO adduct in cytochrome c’" JBIC Journal of Biological Inorganic Chemistry 20(6):949-956. DOI:10.1007/s00775-015-1278-y [RAMAN, UVVis]
- Dworkowski, F.S.N, M. Hough, et al. (2015). "Challenges and solutions for the analysis of in situ, in crystallo microspectrophotometric data" Acta Crystallographica Section D 71(1): 27-35 DOI:10.1107/S1399004714015107 [REVIEW, SOFTWARE]
2014
- Bolze, C. S., R.E. Helbling, et al. (2014). "Human Cellular Retinaldehyde-Binding Protein Has Secondary Thermal 9-cis-Retinal Isomerase Activity." JACS 136(1): 137-146. DOI:10.1021/ja411366w [RAMAN, UV/Vis]
- Kekilli, D., F.S.N. Dworkowski, et al. (2014). "Fingerprinting redox and ligand states in haemprotein crystal structures using resonance Raman spectroscopy." Acta Crystallogr D Biol Crystallogr 70(Pt 5): 1289-1296. DOI:10.1107/S1399004714004039 [RRAMAN]
2013
- Pompidor, G., F.S.N. Dworkowski, et al. (2013). "A new on-axis micro-spectrophotometer for combining Raman, fluorescence and UV/Vis absorption spectroscopy with macromolecular crystallography at the Swiss Light Source." Journal of Synchrotron Radiation 20(5): 765-776. DOI:10.1107/S0909049513016063 [LUMINESCENCE, INSTRUMENTATION]
- Merlino, A., M. R. Fuchs, et al. (2013). "Selective X-ray-induced NO photodissociation in haemoglobin crystals: evidence from a Raman-assisted crystallographic study." Acta Crystallographica Section D-Biological Crystallography 69: 137-140. DOI: 10.1107/S0907444912042229 [RAMAN]
2012
- He, C., M. R. Fuchs, et. al. (2012). "Guanidine-Ferroheme Coordination in the Mutant Protein Nitrophorin 4(L130R)." Angewandte Chemie International Edition 51(18): 4470-4473. DOI: 10.1002/anie.201108691 [UV/Vis]
- Owen, R. L., B. A. Yorke, et al. (2012). "X-ray-excited optical luminescence of protein crystals: a new tool for studying radiation damage during diffraction data collection." Acta Crystallographica Section D 68(5): 505-510. DOI: 10.1107/s0907444912002946 [XEOL]
2011
- Regis Faro, A., P. Carpentier, et al. (2011). "Low-Temperature Chromophore Isomerization Reveals the Photoswitching Mechanism of the Fluorescent Protein Padron." Journal of the American Chemical Society. 133(41): 16362–16365 DOI:0.1021/ja207001y [UV/Vis, LASER INDUCED CHEMISTRY]
- Antonyuk, S. V., M. A. Hough (2011). "Monitoring and validating active site redox states in protein crystals." Biochimica et Biophysica Acta (BBA) - Proteins & Proteomics 1814(6): 778-784. DOI:10.1016/j.bbapap.2010.12.017 [UV/Vis]
- Rajendran, C., F. S. Dworkowski, et al. (2011). "Radiation damage in room-temperature data acquisition with the PILATUS 6M pixel detector." J Synchrotron Radiat 18(Pt 3): 318-328. DOI:10.1107/S090904951100968X [UV/Vis]
2010
- McGeehan, J. E., D. Bourgeois, et al. (2010). "Raman-assisted crystallography of biomolecules at the synchrotron: Instrumentation, methods and applications." Biochim Biophys Acta. DOI:10.1016/j.bbapap.2010.07.021 [REVIEW]
- Hersleth, H. P. and K. K. Andersson (2010). "How different oxidation states of crystalline myoglobin are influenced by X-rays." Biochim Biophys Acta. DOI:10.1016/j.bbapap.2010.07.019 [UV/Vis]
2009
- Owen, R. L., A. R. Pearson, et al. (2009). "A new on-axis multimode spectrometer for the macromolecular crystallography beamlines of the Swiss Light Source." J. Synchrotron Rad. 16(Pt 2): 173-182. DOI:10.1107/S0909049508040120 [INSTRUMENTATION]
- Pearson, A. R. and R. L. Owen (2009). "Combining X-ray crystallography and single-crystal spectroscopy to probe enzyme mechanisms." Biochemical Society Transactions 037(2): 378-381. DOI:10.1042/bst0370378 [REVIEW, INSTRUMENTATION]