The PSI Electrochemistry Laboratory studies almost all aspects of electrochemical energy storage and conversion.
Lab News & Scientific Highlights
Best practices for harnessing operando X-ray absorption spectroscopy in electrocatalytic water splitting studies
X-ray absorption spectroscopy (XAS) has found applications in a range of fields including materials, physics, chemistry, biology and earth science. XAS can probe the local electronic and geometric structure, such as the average oxidation state, coordination environment and interatomic distances, surrounding an element of interest. Thus, XAS is a valuable tool to inform catalyst design by tracking catalyst evolution under operating conditions, for example, via providing dynamic snapshots of the essential information.
Converting the CHF3 greenhouse gas into LiF coating for high-voltage cathode materials toward high-energy density Li-ion batteries
The instability and the fading of high voltage cathode materials above 4.3 V remains a major challenge for the next generation of high energy density Li-ion batteries. Here, we present a facile, environmentally friendly, cost effective and scalable method to address this problem by uniformly fluorinating the surface of cathode materials with CHF3, a mild fluorinating agent but a potent greenhouse gas. CHF3 is successfully transformed into ~2 nm LiF homogenous layer covering the surface of layered-oxide cathode materials.
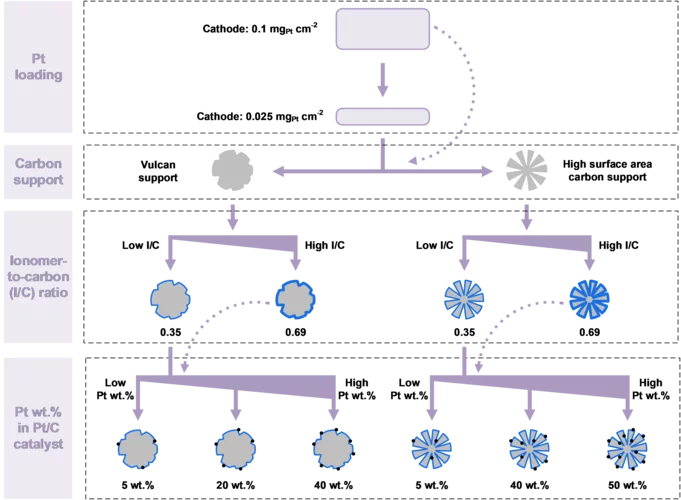
Hydrogen Electrode for Membrane Water Electrolyzers with Low Gas Crossover
Proton exchange membrane (PEM) water electrolyzer are considered a for the Energy Transition to produce green hydrogen for fuel cell-based mobility, industrial processes, and seasonal storage. Platinum group metals (PGMs) are conventionally used as catalysts for electrode reactions due to their outstanding catalytic activity and chemical stability in the harsh acidic environment of the cell. Commercial carbon-supported platinum (Pt/C) electrocatalysts remains a state-of-the-art choice for the hydrogen evolution reaction (HER) on the cathode side of the cell. While a high Pt loading between 0.5 and 1.0 mgPt/cm2 is commonly used today, a reduction of the Pt loading to below 0.05 mgPt/cm2 is desired to reduce the cost of PGM usage in megawatt-scale PEM water electrolysis systems. In addition, in connection with the trend towards the use of thinner membranes (<0.1 mm), gas crossover through the membrane from the cathode to the anode side can lead to the formation of an explosive gas mixture in the anode product stream. In this study, we varied the design parameters for the cathode catalyst layer to reduce the Pt loading to 0.025 mg/cm2 while at the same time minimizing the rate of hydrogen crossover to the anode.