Proton exchange membrane (PEM) water electrolyzer are considered a for the Energy Transition to produce green hydrogen for fuel cell-based mobility, industrial processes, and seasonal storage. Platinum group metals (PGMs) are conventionally used as catalysts for electrode reactions due to their outstanding catalytic activity and chemical stability in the harsh acidic environment of the cell. Commercial carbon-supported platinum (Pt/C) electrocatalysts remains a state-of-the-art choice for the hydrogen evolution reaction (HER) on the cathode side of the cell. While a high Pt loading between 0.5 and 1.0 mgPt/cm2 is commonly used today, a reduction of the Pt loading to below 0.05 mgPt/cm2 is desired to reduce the cost of PGM usage in megawatt-scale PEM water electrolysis systems. In addition, in connection with the trend towards the use of thinner membranes (<0.1 mm), gas crossover through the membrane from the cathode to the anode side can lead to the formation of an explosive gas mixture in the anode product stream. In this study, we varied the design parameters for the cathode catalyst layer to reduce the Pt loading to 0.025 mg/cm2 while at the same time minimizing the rate of hydrogen crossover to the anode.
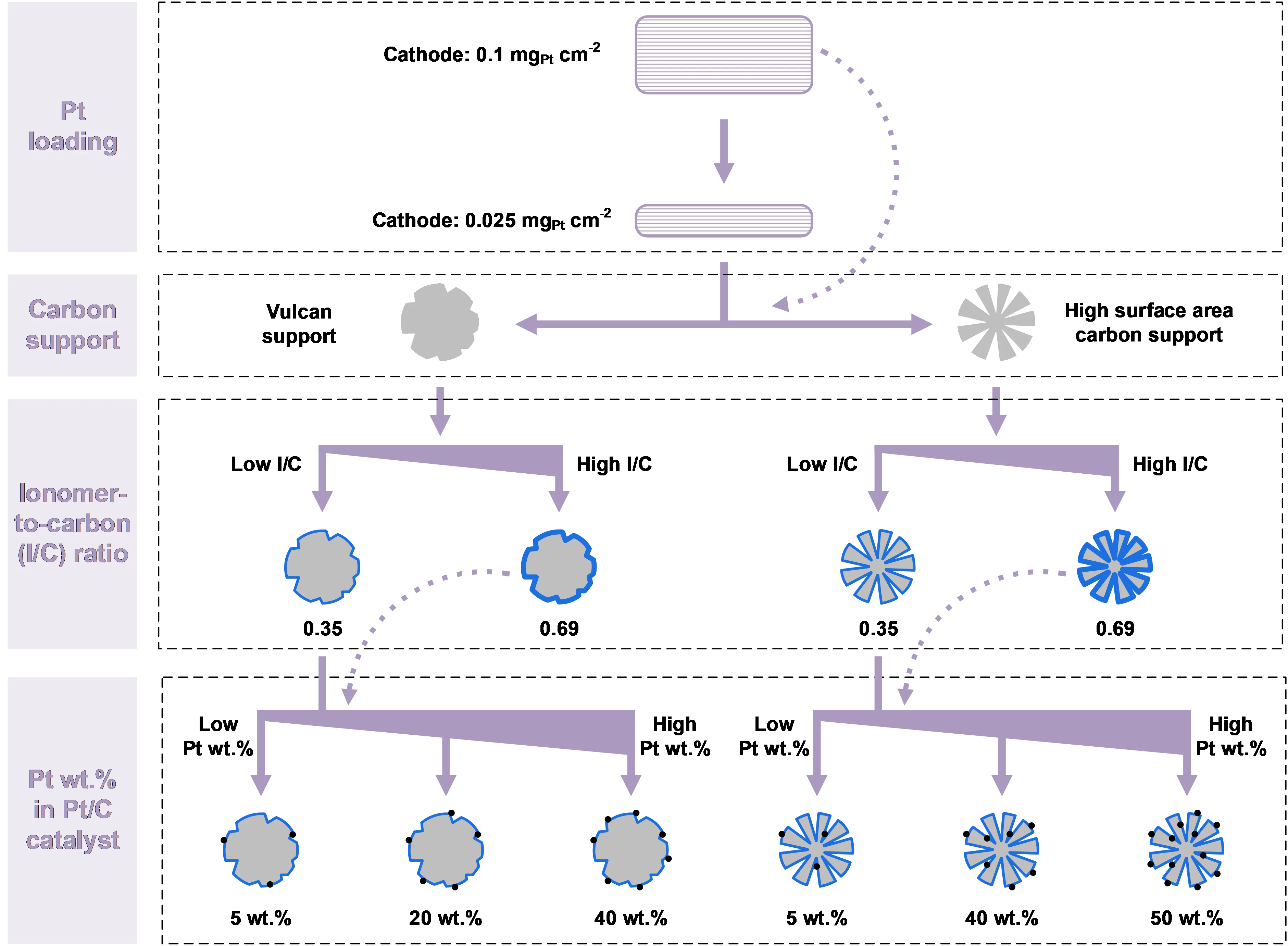
We present a detailed study of the cathode catalyst layer to concurrently decrease the loading of Pt and minimize the rate of hydrogen crossover, through the analysis of cathode catalyst layers featuring a variety of structural parameter combinations. A four-fold reduction of Pt was achieved from 0.1 mg/cm2 to 0.025 mg/cm2. The Pt/C catalyst was chosen from two types of the carbon support: Vulcan carbon (~250 m2/g) and high surface area carbon (HSAC), and from a range of Pt wt.% in Pt/C between nominally 5 and 50 wt.%. Two levels of the ionomer content, represented by an ionomter-to-carbon (I/C) mass ratio of 0.35 and 0.69, were employed. Figure 1 shows an overview of the different cathode catalyst layer compositions investigated. Electrolysis single cell measurements were carried out and the electrochemical performance of the cell containing the various cathode configurations was compared. A decrease of Pt wt.% in Pt/C, I/C ratio or an increase of the Pt loading was proven to be typically beneficial in decreasing the rate of hydrogen permeation. The analysis of the H2 crossover indicates that a lower Pt-wt% and I/C ratio generally contribute to a reduced hydrogen permeation rate. In contrast, a decrease in Pt loading may lead to an opposite effect, especially in cathodes using Vulcan carbon-supported catalysts. At a Pt loading of 0.025 mg/cm2, the cathode of 40 wt.% Pt on HSAC support with an I/C ratio of 0.35 shows the highest calculated hydrogen mass transfer coefficient (22 mm/s), which is seen as the optimal configuration. These findings help to provide guidance for the design criteria of the next-generation PEM water electrolysis cells.
Contact
Dr. Lorenz Gubler
Research Group Leader, Membranes & Electrochemical Cells group
Paul Scherrer Institut PSI, 5232 Villigen PSI, Switzerland
lorenz.gubler@psi.ch
Original Publications
Cathode Catalyst Layer Design in PEM Water Electrolysis toward Reduced Pt Loading and Hydrogen Crossover
Zheyu Zhang, Axelle Baudy, Andrea Testino, Lorenz Gubler
ACS Appl. Mater. Interfaces 16 (2024) 23265-23277
DOI: 10.1021/acsami.4c01827
Acknowledgement to Funding Agency
Swiss Federal Office of Energy (grant number SI/502174).