Mechano-Genomics & AI Group at PSI, jointly with ETH-Zurich
Our bodies contain trillions of cells, each uniquely organized with the same DNA to enable cell-type-specific gene expression programs. The mechano-genomic regulation of DNA packaging (chromatin) plays a crucial role in maintaining cell states, and disruptions in this packaging during cellular aging can lead to various age-related diseases. Despite the significance, the underlying mechanisms remain poorly understood. Our group focuses on understanding the mechano-genomics of cellular aging and exploring how aging cells can be reprogrammed and rejuvenated for cell-based therapies. Additionally, we develop AI-driven quantitative single-cell chromatin imaging biomarkers, which serve as fingerprints for cellular health and disease. Our research program is highly interdisciplinary, employing advanced techniques in light microscopy, microfabrication, functional genomics, theoretical modeling, and machine learning. We collaborate closely with Caroline Uhler’s group at MIT/Broad Institute on the computational front. Building on our current strengths, our research focuses on two major research themes, and a public health project and enabling technology transfer:
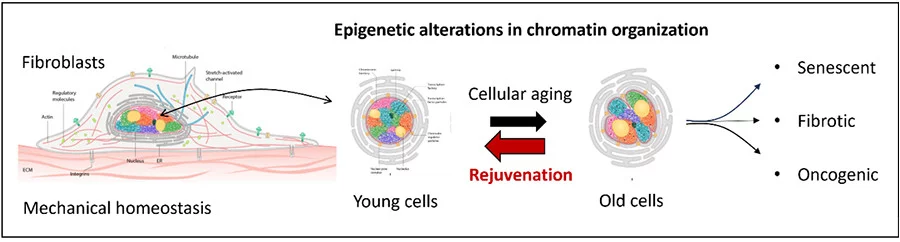
Theme 1: Mechano-Genomics of Cellular Aging & Rejuvenation
Aging is an inevitable process that prompts the exploration of various anti-aging strategies. Notably, landmark discoveries related to chromatin reprogramming through exogenous factors have shown considerable promise. However, significant challenges remain, particularly regarding oncogenic cell-state transitions that can arise from such reprogramming in cell-based therapies. In this context, we recently demonstrated that mechano-chemical signals, independent of exogenous factors, can induce partial programming and rejuvenation of aging fibroblasts. Our ongoing research employs a combination of fluorescence imaging, genome-wide analysis, RNA sequencing, interventional screens, and theoretical modeling to identify the molecular pathways involved in the mechanical adaptation and rejuvenation of aging cells and those associated with age-related diseases. Additionally, we have shown that implanting partially reprogrammed aged fibroblasts into engineered aged skin models leads to enhanced tissue regeneration and repair. Our primary focus moving forward is to evaluate cell health in aging tissues and cell-based therapy models. We will integrate single-cell chromatin imaging biomarkers with spatial omics to quantitatively analyze the aging tissue microenvironment. This approach will enable us to explore how mechanical reprogramming of stromal cells and PBMCs, and their implantation, can create effective cell therapy models that reverse age-related changes in tissue architecture and function. We will specifically target wound healing in aged human skin, with potential applications in cancer and fibrosis.
Theme 2: AI-based Chromatin Imaging Biomarkers for Health & Disease
Disease initiation and progression lead to the secretion of various signaling factors into the local tissue microenvironment, resulting in significant reorganization of tissue mechanical architecture. Using tissue microarrays, we recently demonstrated that our single-cell chromatin biomarkers can uniquely trace cell-state transitions associated with breast tumor progression and neurodegeneration. Since disease secretome signals are also released into the bloodstream, we hypothesized that the chromatin states of peripheral blood mononuclear cells could differentiate between healthy individuals and those with disease. Through collaborations with the Center for Proton Therapy at PSI and the local Health Center in Brugg, we have successfully shown that subtle alterations in blood cell chromatin states, induced by disease and stress secretome signals, can serve as robust biomarkers for aging-related diseases, including cancer and metabolic disorders. Our ongoing research is investigating the mechanistic basis of detecting tumor microenvironmental signals through PBMC chromatin states. In the next phase, leveraging our chromatin imaging atlas of PBMCs, we will assess how early we can detect various types of cancers, chronic stress, and neurodegenerative secretome signals in the blood using our chromatin biomarkers. Additionally, we will investigate whether these blood-based chromatin biomarkers can effectively track the efficacy of various therapeutic models, including Proton Therapy at PSI. Collectively, this initiative will open new avenues for early disease diagnostics and enhance the monitoring of therapeutic efficacy in personalized and precision medicine.
Public Health Project: "AI-based Chromatin Imaging Atlas of Blood Cells during Healthy Aging"
Aging is associated with a progressive decline in cellular function, influenced by changes in nuclear mechanotransduction and 3D chromatin states that affect genomic programs. Systematic evaluation of these declines could uncover valuable biomarkers for healthy aging. Our recent studies show that AI-based analysis of single-cell chromatin images provides exceptional sensitivity in detecting genomic alterations linked to health and disease. Building on this foundation and recognizing the critical role of peripheral blood mononuclear cells (PBMCs) in immune response, along with their easy accessibility through liquid biopsies, we are creating a comprehensive chromatin imaging atlas of PBMCs from healthy human donors spanning diverse ages and genders, in collaboration with major blood banks. Additionally, we are developing a microfluidic device integrated with a miniaturized fluorescence microscope that attaches to a mobile phone to purify and image the chromatin states of PBMCs. This innovative, cost-effective approach will enable scalable monitoring of healthy aging through PBMC chromatin imaging biomarkers. We envision the imaging atlas as an open-access resource for both basic researchers and clinicians.
Technology transfer
Our research program in mechano-genomics and AI has culminated in the establishment of FOCAL Biosciences, a venture launched in collaboration with Apollo Ventures and PSI/ETH Zurich. FOCAL Biosciences aims to harness our expertise to create a cutting-edge drug discovery platform that utilizes mechano-genomic principles underlying fibroblast cell-state transitions, combined with imaging-AI-based chromatin biomarkers as reliable phenotypic readouts.
Teaching at D-HEST, ETH Zurich:
- Spring (yearly): Mechano-Genomics in Health & Disease
- Fall (yearly): Cellular Aging