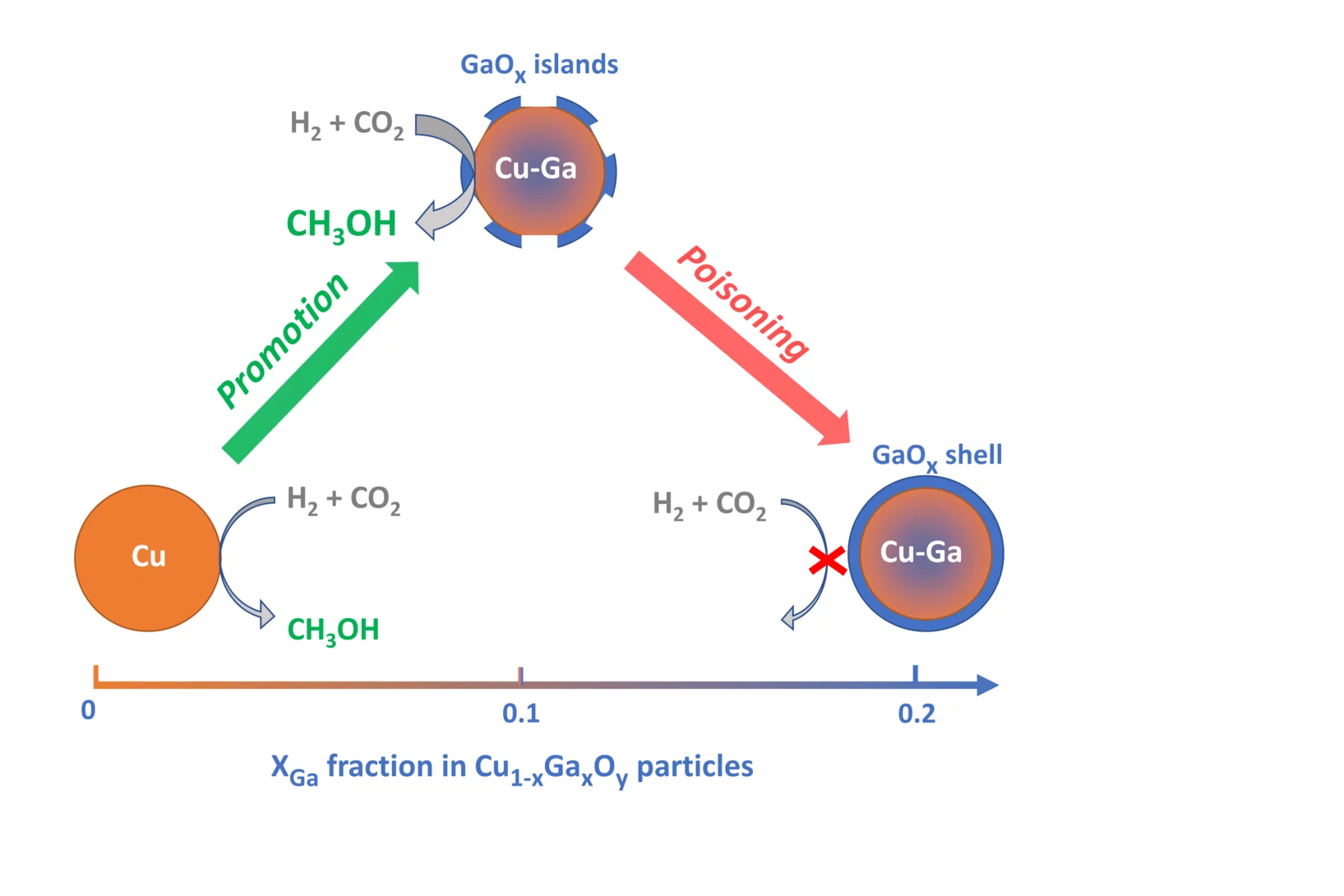
Promotion versus Poisoning in Copper–Gallium-Based CO2-to-Methanol Hydrogenation Catalysts
Cu–Ga-based CO2-to-methanol hydrogenation catalysts display a range of catalytic performance, depending on their preparation. Here, we investigated how the Ga/Cu ratio and Ga speciation affect the catalytic activity. Using surface organometallic chemistry, we prepared a series of silica-supported 3–6 nm Cu1–xGaxOy nanoparticles with a range of xGa. The materials display a volcano-type activity behavior, where methanol formation is promoted when xGa < 0.13–0.18 and is suppressed at higher values, indicating a poisoning of the catalysts. In situ X-ray absorption spectroscopy and in situ infrared spectroscopy helped to understand the structure-activity relationship.
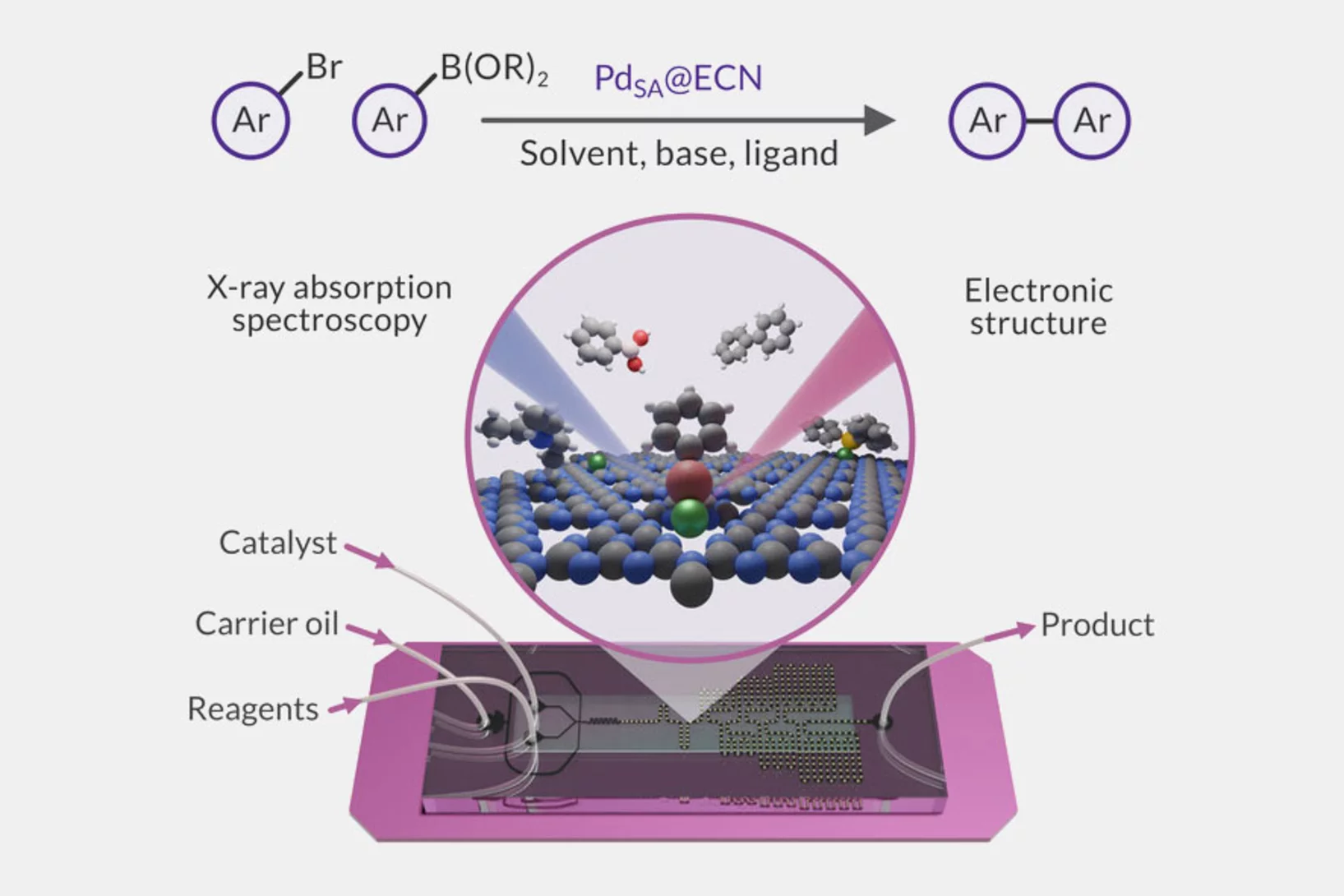
Microfluidic platform for in situ characterization of heterogenous catalysts
A deep understanding of active site architectures during surface-catalyzed reactions is a crucial step for the design of recyclable heterogeneous catalysts for organic synthesis. In this work, a droplet-based microfluidic setup was developed and applied to perform Suzuki-Miyaura coupling over heterogenous single-atom Pd-catalyst.
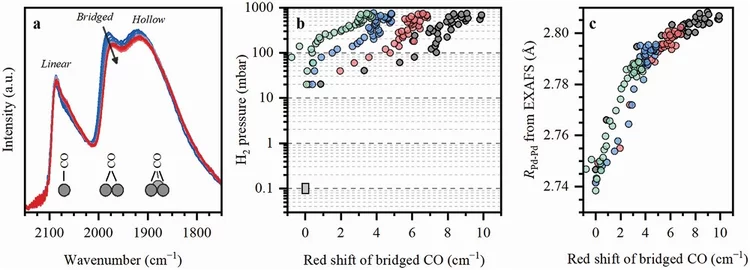
Machine Learning for Quantitative Structural Information from Infrared Spectra: The Case of Palladium Hydride
Infrared spectroscopy (IR) is a widely used technique enabling to identify specific functional groups in the molecule of interest based on their characteristic vibrational modes or the presence of a specific adsorption site based on the characteristic vibrational mode of an adsorbed probe molecule. The interpretation of an IR spectrum is generally carried out within a fingerprint paradigm by comparing the observed spectral features with the features of known references or theoretical calculations. This work demonstrates a method for extracting quantitative structural information beyond this approach by application of machine learning (ML) algorithms.
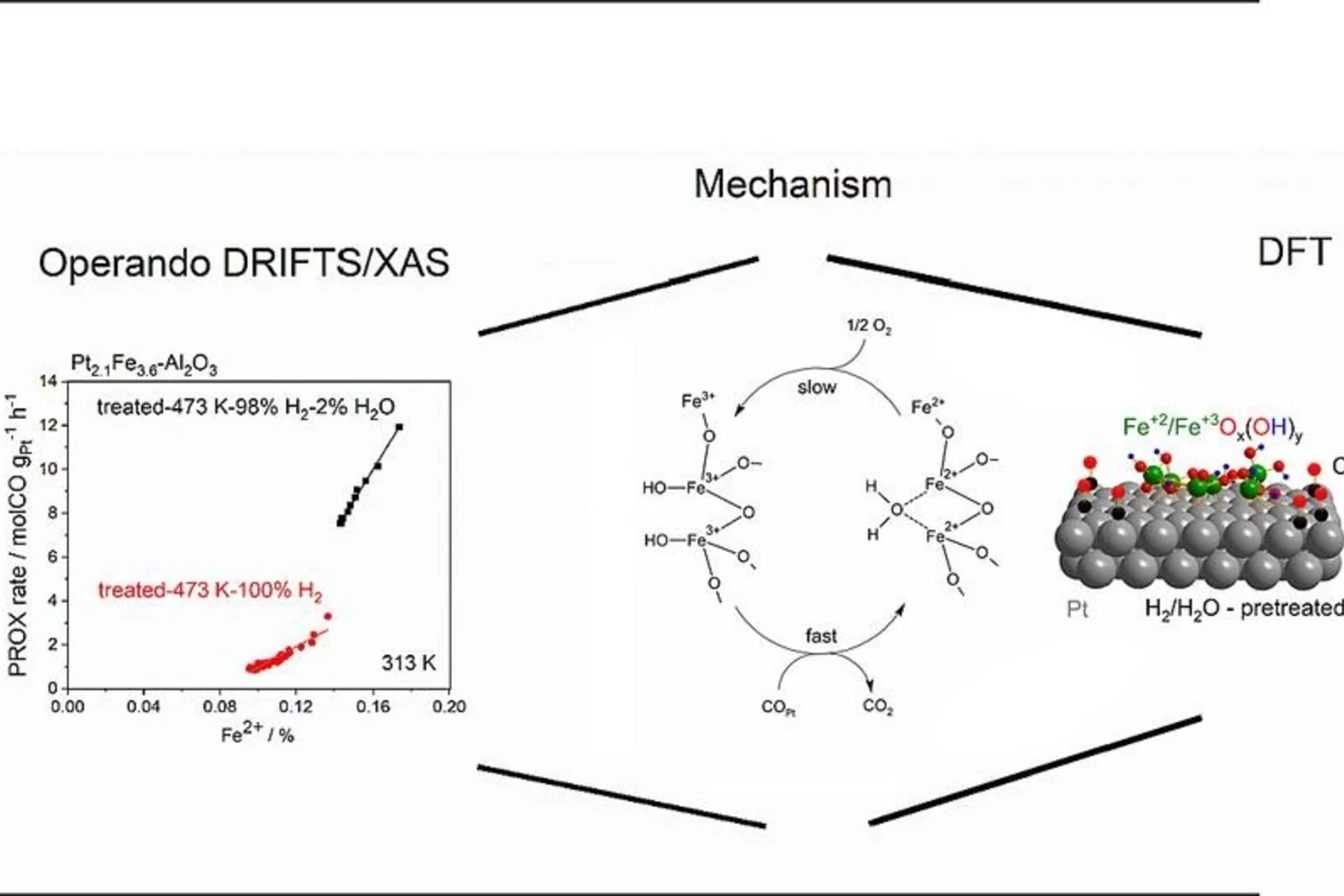
Water-assisted generation of catalytic interface: The case of interfacial Pt-FeOx(OH)y sites active in preferential carbon monoxide oxidation
Pt-FeOx(OH)y interface with enhanced activity for PROX is generated via strong metal-support interaction. Increase in the degree of hydroxylation of Pt-FeOx(OH)y interface enhances the rate of PROX mediated by active Fe2+ species.
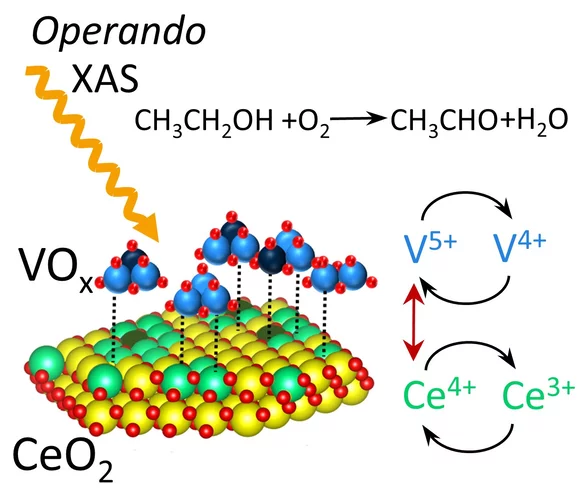
Activity Trend Origin of Ethanol Oxidative Dehydrogenation over VOx/CeO2
Using operando time-resolved X-ray absorption spectroscopy, we investigated the origin of volcano-shaped ethanol oxidative dehydrogenation activity trend of VOx/CeO2 catalysts as a function of VOx surface coverage. Vanadium and cerium synergistically change their oxidation states during the catalytic cycle. The catalytic activity correlates with the concentration of reversible Ce4+/3+species.
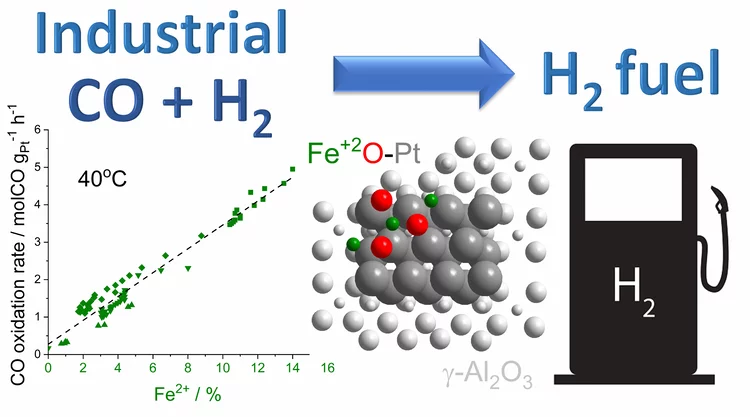
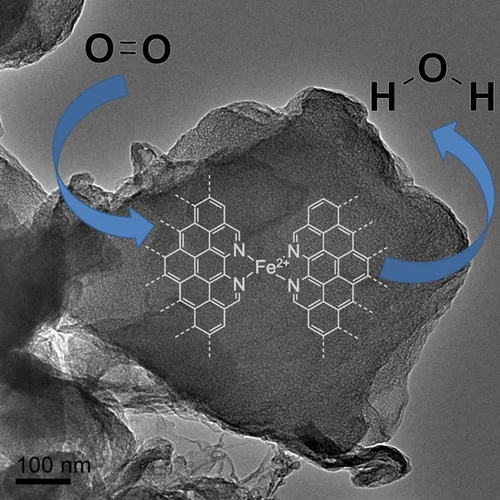
Platinum-Iron(II) Oxide Sites Directly Responsible for Preferential Carbon Monoxide Oxidation at Ambient Temperature: An Operando X-ray Absorption Spectroscopy Study
Operando X-ray absorption spectroscopy revealed a linear correlation between the amount of oxidic Fe2+ and the ambient temperature activity of Pt−FeOx preferential carbon monoxide oxidation catalysts. The hydrogen prereduction temperature and pressure determines the amount of active Fe2+ sites for alumina- and silica-supported Pt−Fe catalysts. Catalyst deactivation is linked with the oxidation of these sites.
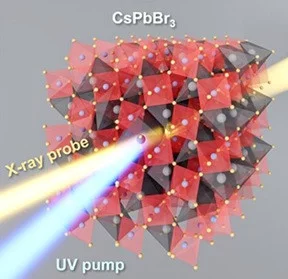
Quantifying Photoinduced Polaronic and Thermal Distortions in Inorganic Lead Halide Perovskite Nanocrystals
The development of next-generation perovskite-based optoelectronic devices relies critically on the understanding of the interaction between charge carriers and the polar lattice in out-of-equilibrium conditions. While it has become increasingly evident for CsPbBr3 perovskites that the Pb–Br framework flexibility plays a key role in their light-activated functionality, the corresponding local structural rearrangement has not yet been unambiguously identified. In this work the photoinduced lattice changes were investigated using combination of time-resolved and temperature-dependent studies at Br K and Pb L3 X-ray absorption edges and ab initio simulations.
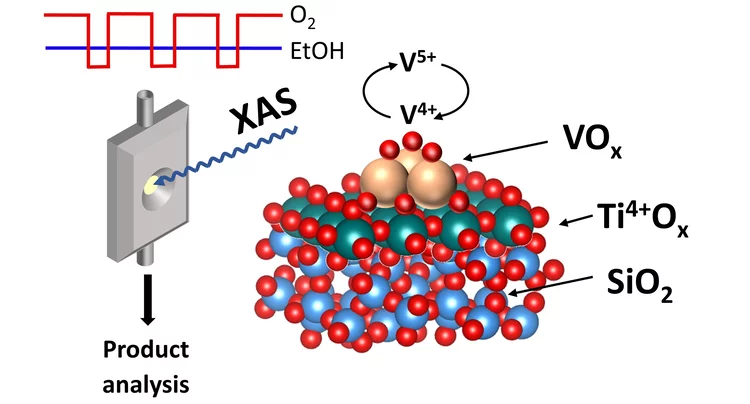
Redox Dynamics of Active VOx Sites Promoted by TiOx during Oxidative Dehydrogenation of Ethanol Detected by Operando Quick XAS
Operando time-resolved V and Ti K-edge X-ray absorption near-edge spectroscopy, coupled with a transient experimental strategy, quantitatively showed that the formation of acetaldehyde over 5% V2O5/15% TiO2/SiO2 is kinetically coupled to the formation of a V4+ intermediate, while the formation of V3+ is delayed and 10–70 times slower. The low-coordinated nature of various redox states of VOx species (V5+, V4+, and V3+) in the 5% V2O5/15% TiO2/SiO2 catalyst is confirmed using the extensive database of V K-edge XANES spectra of standards and specially synthesized molecular crystals.
Exploring the role of structural distortions to obtain Cu photosensitizer with thousand times longer excited state lifetime
Cu diimine complexes present a noble metal free alternative to classical Ru, Re, Ir and Pt based photosensitizers in solution photochemistry, photoelectrochemical or dye-sensitized solar cells. Optimization of these dyes requires an understanding of factors governing the key photochemical properties: excited state lifetime and emission quantum yield. Using pump-probe XAS and DFT calculations we have explored the involvement of exciplex formation in the deactivation of the photoexcited state.
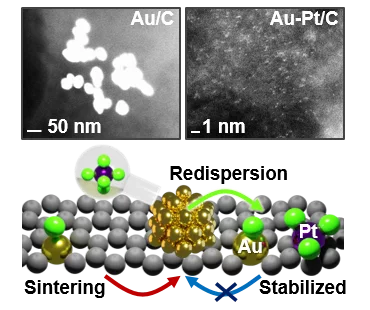
Sustainable Synthesis of Bimetallic Single Atom Gold-Based Catalysts with Enhanced Durability in Acetylene Hydrochlorination
Platinum chloride in aqueous solution promotes the dispersion of large gold nanoparticles (>70 nm) on carbon carriers into single atoms, forming bimetallic single-atom catalysts with improved resistance against sintering at temperatures up to 800 K and under the harsh reductive reaction conditions of acetylene hydrochlorination, leading to improved lifetime in this reaction. To rationalize these observations, this study, led by ETH Zurich, utilized X-ray adsorption spectroscopy conducted at the SuperXAS beamline of the SLS to provide insights into the degree of gold dispersion and the structure of the isolated metal sites in the bimetallic catalysts.
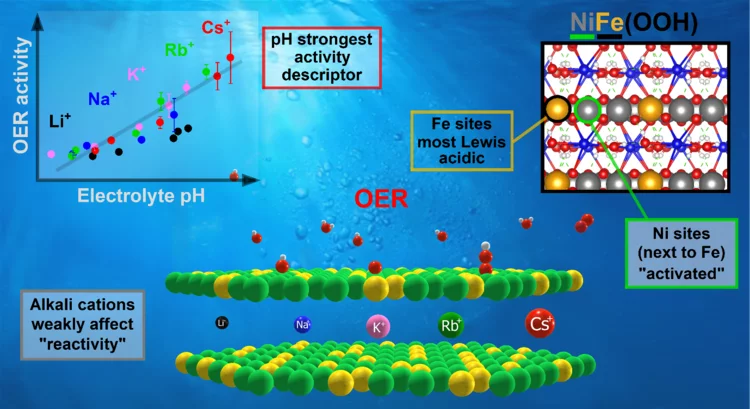
Key activity descriptors of nickel-iron oxygen evolution electrocatalysts in the presence of alkali metal cations
Ni-Fe oxyhydroxide is among the most active oxygen evolution electrocatalysts. Electrolyte alkali metal cations modify the activity and reaction intermediates, however, the exact mechanism is at question due to unexplained deviations from the cation size trend. Our X-ray absorption spectroelectrochemical results show that the OER activity follows the variations in .electrolyte pH rather than a specific cation. Our DFT-based reactivity descriptors confirm the conclusions of an indirect pH effect.
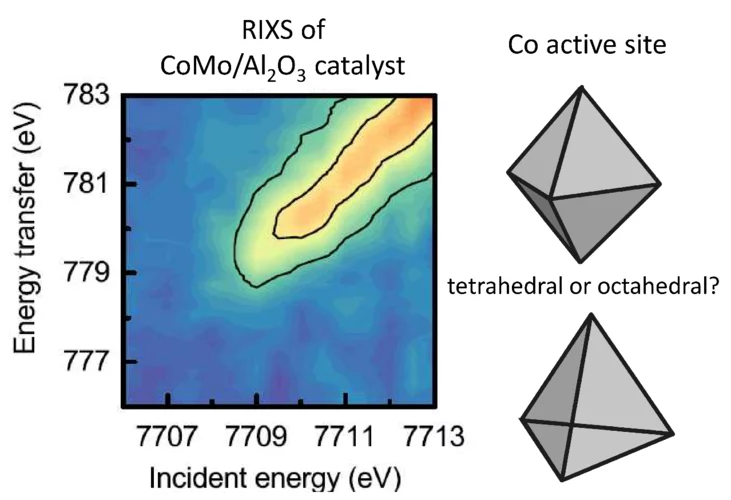
The structure of active sites of CoMo/Al2O3 catalysts determined by RIXS spectroscopy.
A fundamental understanding of the active sites in technical CoMo/ Al2O3 catalysts is crucial to improve the production of clean transportation fuels by hydrodesulfurization (HDS), which removes sulfur from fossil fuels. Sulfur dioxide, resulting from fossil fuel combustion, is one of the main causes for acid rain. In situ X-ray spectroscopic experiments at the SuperXAS beamline of the SLS provided insight in the structure and number of active sites (“Co−Mo−S”) in sulfided CoMo/ Al2O3 catalysts. When the Co to Mo ratio is less than 0.1, cobalt forms isolated sites on the MoS2 phase, where the cobalt promoter atoms are in centrosymmetric octahedral coordination with six-sulfur ligands.
Taking a snapshot of the triplet excited state of an OLED organometallic luminophore using X-rays
In this work, the complementarity of pump-probe experiments at SwissFEL (ALVRA endstation) and at synchrotrons (SuperXAS beamline of SLS and ID09 of ESRF) is used to investigate the triplet excited state of Cu OLED materials. Details about the charge transfer and structural rearrangements in the excited state of this material are revealed and obtained data can be used to verify computational methods for rational development of new OLED materials.
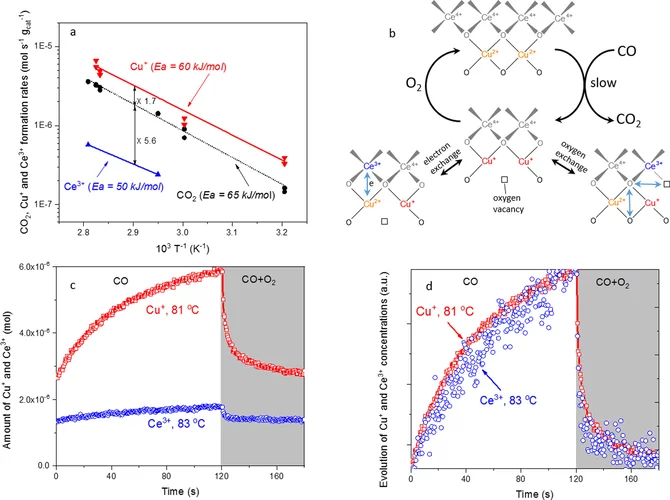
Elucidating the Oxygen Activation Mechanism on Ceria-Supported Copper-Oxo Species Using Time-Resolved X-ray Absorption Spectroscopy
We monitored the dynamic structure of the active sites in a catalyst containing highly dispersed copper-oxo species on ceria during low-temperature CO oxidation using time-resolved X-ray absorption spectroscopy. We quantitatively demonstrate that the CO oxidation mechanism below 90 °C involves an oxygen intermediate strongly bound to the active sites as well as the redox activity of Cu2+/Cu+ and Ce4+/Ce3+ couples.
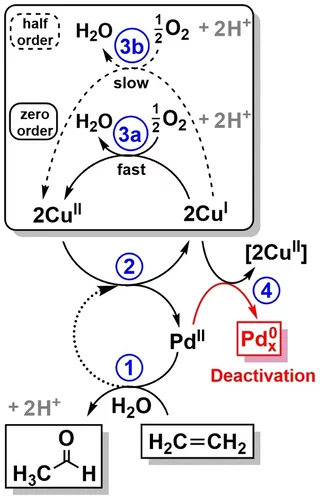
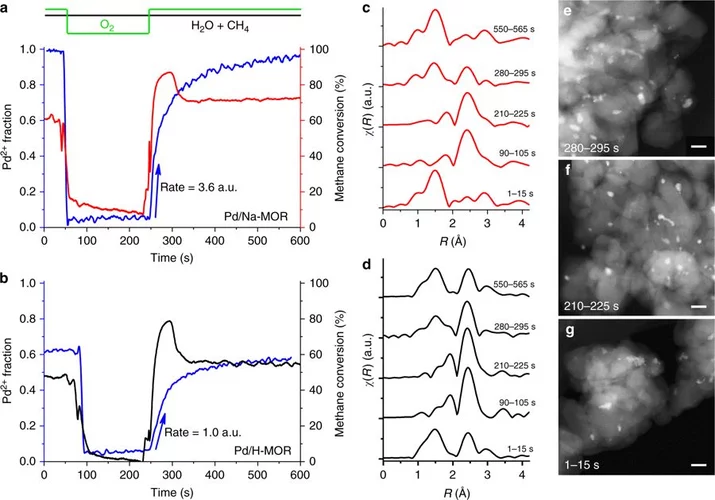
Elucidating the mechanism of heterogeneous Wacker oxidation over Pd-Cu/zeolite Y by transient XAS
Unlike the homogeneous Wacker process, the understanding of the mechanism of the heterogeneous system has long remained to be superficial. Here the authors investigated the mechanism of heterogeneous Wacker oxidation over Pd-Cu/zeolite Y through transient XAS coupled with kinetic studies and chemometric analysis.
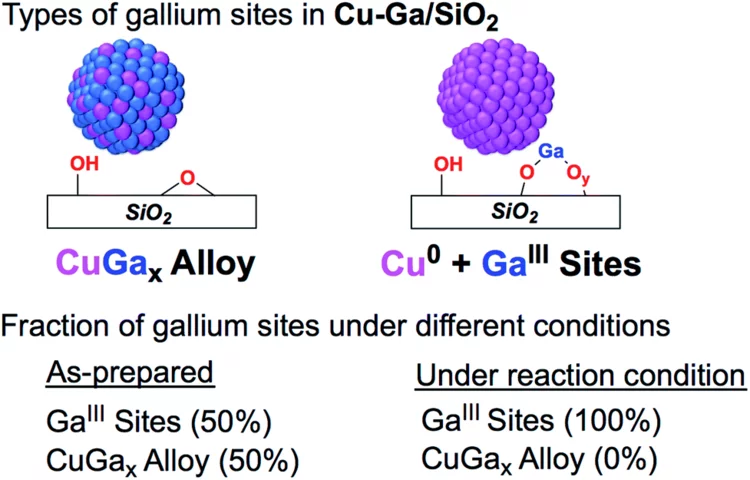
Enhanced CH3OH selectivity in CO2 hydrogenation using Cu-based catalysts generated via SOMC from GaIII single-sites
Small and narrowly distributed nanoparticles of copper alloyed with gallium supported on silica containing residual GaIII sites can be obtained via surface organometallic chemistry. This material is highly active and selective for CO2 hydrogenation to CH3OH. In situ X-ray absorption spectroscopy shows that gallium is oxidized under reaction conditions while copper remains as Cu0.
Structural selectivity of supported Pd nanoparticles for catalytic NH3 oxidation resolved using combined operando spectroscopy
The link between Pd nanoparticle structure and surface reactivity for NH3 abatement was found using operando X-ray absorption fine structure spectroscopy, diffuse reflectance infrared Fourier-transformed spectroscopy and on-line mass spectrometry.
Fe-Based O2-Reduction Catalysts Synthesized Using Na2CO3 as a Pore-Inducing Agent
This work presents a new approach for synthesizing Fe-based oxygen reduction reaction (ORR) catalysts using sodium carbonate (Na2CO3) as an inexpensive but effective pore-inducing agent offering microporosity control.
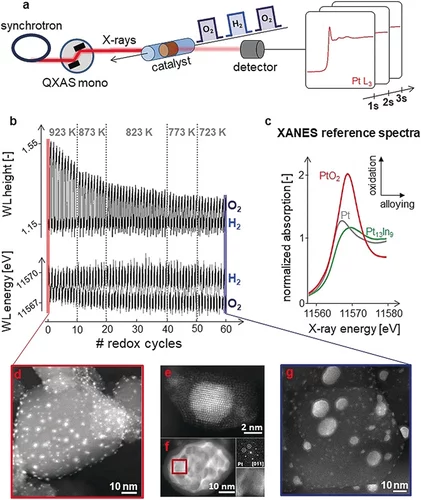
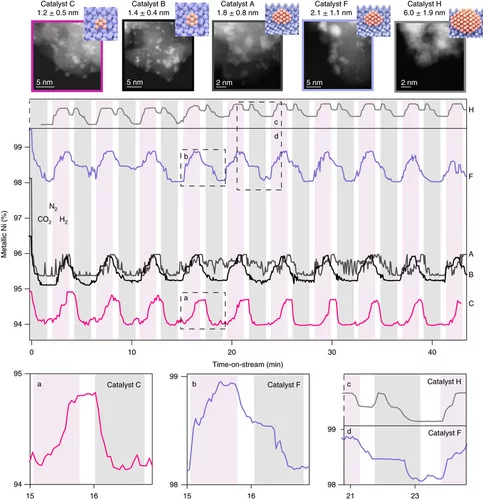
Kinetics of Lifetime Changes in Bimetallic Nanocatalysts Revealed by Quick X‐ray Absorption Spectroscopy
The different reaction steps involved in repeated Pt13In9 segregation‐alloying are identified by XAS and kinetically characterized at the single‐cycle level.
The SLS congratulates Ronald Frahm for receiving the IXAS outstanding achievement Award 2018
The highest award of the international X-ray absorption spectroscopy (IXAS) society, the Edward Stern Outstanding Achievement Award, was presented to Prof. Ronald Frahm during the tri-annual IXAS meeting in Kraków, Poland in July 2018.
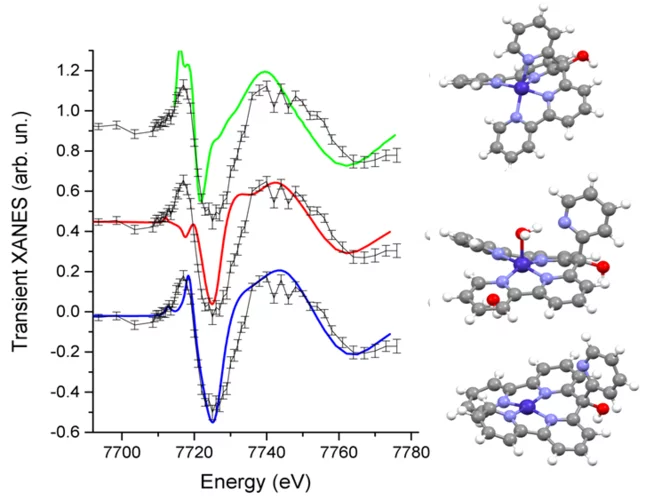
Structure of the Co(I) Intermediate of a Cobalt Pentapyridyl Catalyst for Hydrogen Evolution Revealed by Time‐Resolved X‐ray Spectroscopy
The mechanism of hydrogen evolution by cobalt polypyridyls catalysts is investigated. Pump-probe X‐ray absorption spectra measured at SuperXAS in the microsecond time range indicate that the pendant pyridine dissociates from the cobalt in the intermediate Co(I) state. This opens the possibility for pyridinium to act as an intramolecular proton donor, which can be used for the development of efficient catalysts.
Stable complete methane oxidation over palladium based zeolite catalysts
Using targeted synthesis and in situ characterization a palladium catalyst with improved stability against sintering during methane oxidation was prepared.
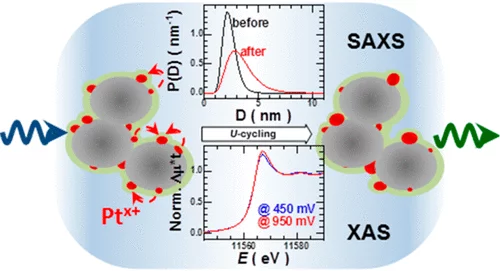
Combining SAXS and XAS To Study the Operando Degradation of Carbon-Supported Pt-Nanoparticle Fuel Cell Catalysts
In the last two decades, small-angle X-ray scattering (SAXS) and X-ray absorption spectroscopy (XAS) have evolved into two well-established techniques capable of providing complementary and operando information about a sample’s morphology and composition, respectively. Considering that operation conditions can often lead to simultaneous and related changes in a catalyst’s speciation and shape, herein we introduce a setup that combines SAXS and XAS in a configuration that allows optimum acquisition and corresponding data quality for both techniques.
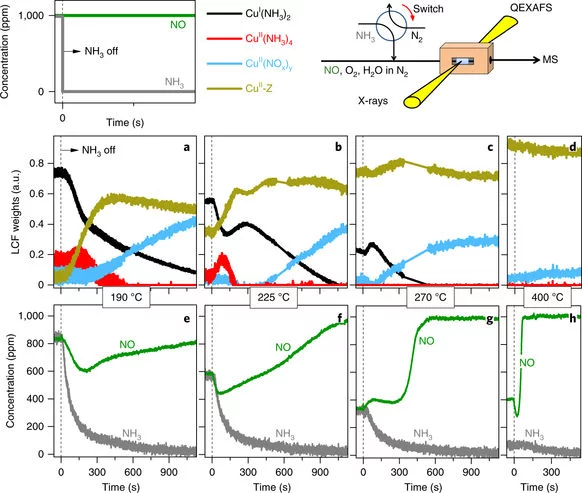
Time-resolved copper speciation during selective catalytic reduction of NO on Cu-SSZ-13
Through the combination of time-resolved X-ray absorption spectroscopy and transient experimentation, we were able to capture an ammonia inhibition effect on the rate-limiting copper re-oxidation at low temperature.
Unravelling structure sensitivity in CO2 hydrogenation over nickel
Using a unique set of well-defined silica-supported Ni nanoclusters (1–7 nm) and advanced characterization methods it was proved how structure sensitivity influences the mechanism of catalytic CO2 reduction, the nature of which has been long debated.
Nanomaterial helps store solar energy: efficiently and inexpensively
By combining a scalable cutting-edge synthesis method with time-resolved X-ray absorption spectroscopy measurements, it was possible to capture the dynamic local electronic and geometric structure during realistic operando conditions for highly active OER perovskite nanocatalysts.
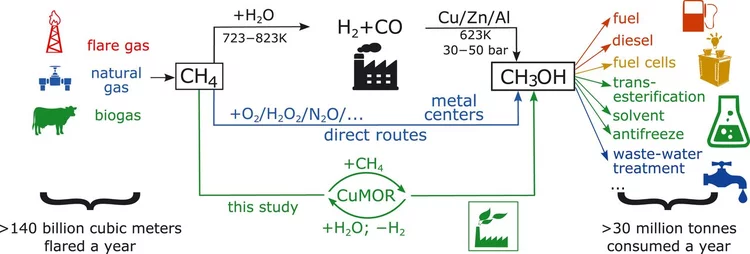
Selective anaerobic oxidation of methane enables direct synthesis of methanol
On the basis of in situ x-ray absorption spectroscopy, infrared spectroscopy, and density functional theory calculations, it was proposed a mechanism involving methane oxidation at Cu II oxide active centers, followed by Cu I reoxidation by water with concurrent formation of hydrogen.
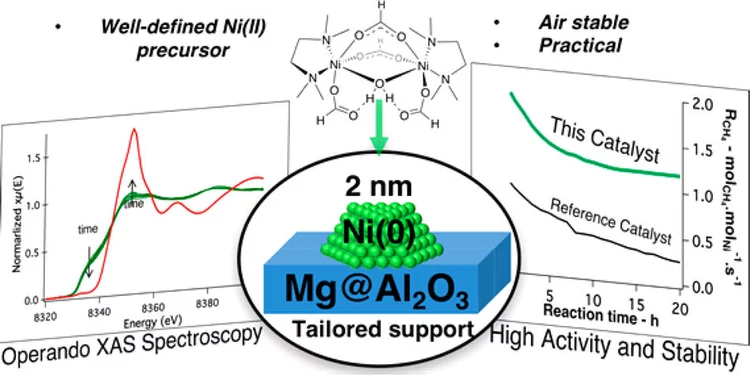
Molecularly Tailored Nickel Precursor and Support Yield a Stable Methane Dry Reforming Catalyst with Superior Metal Utilization
The superior performance of molecularly tailored methane dry reforming catalyst resulted in a maximization of the amount of accessible metallic nickel in the form of small nanoparticles preventing coke deposition. Operando X-ray absorption near-edge structure spectroscopy confirms that deactivation largely occurs through the migration of Ni into the support.
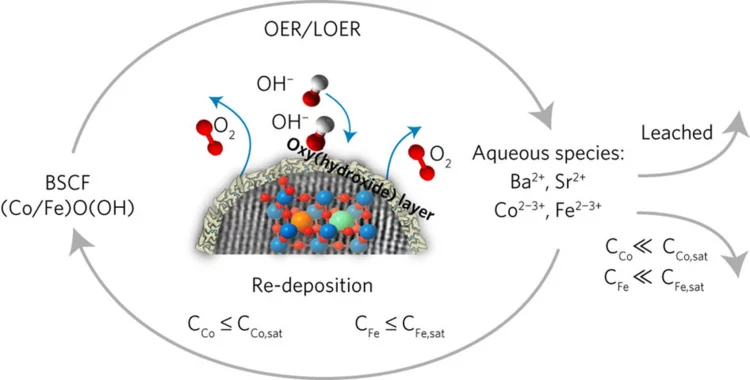
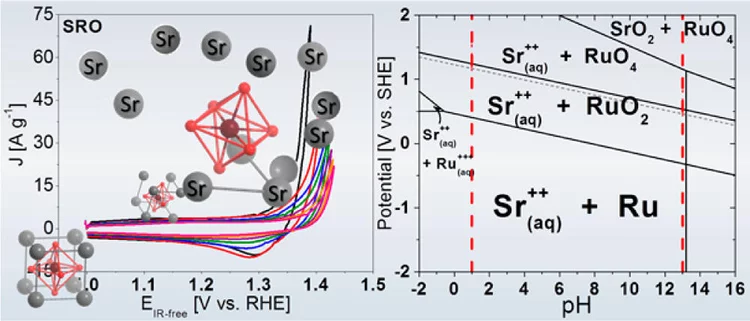
Unraveling Thermodynamics, Stability, and Oxygen Evolution Activity of Strontium Ruthenium Perovskite Oxide
Ru-based perovskites, i.e. SrRuO3 and LaRuO3, have been predicted as active perovskites to exhibit a particularly high oxygen evolution reaction activity. We highlight that understanding the origin of stability under a real operating environment is absolutely essential for the design of a sustainable electrocatalyst with optimal balance between activity and stability.
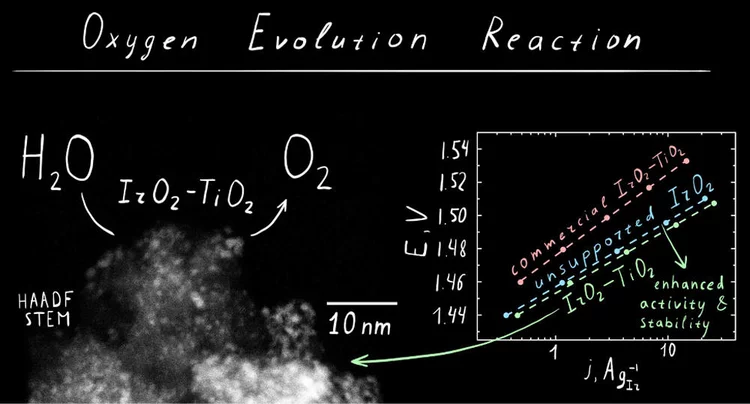
IrO2‑TiO2: A High-Surface-Area, Active, and Stable Electrocatalyst for the Oxygen Evolution Reaction
We have developed a synthetic approach to highsurface-area chlorine-free iridium oxide nanoparticles dispersed in titania (IrO2-TiO2), which is a highly active and stable OER catalyst in acidic media. Operando X-ray absorption studies demonstrate the evolution of the surface species as a function of the applied potential, suggesting the conversion of the initial hydroxo surface layer to the oxo-terminated surface via anodic oxidation.