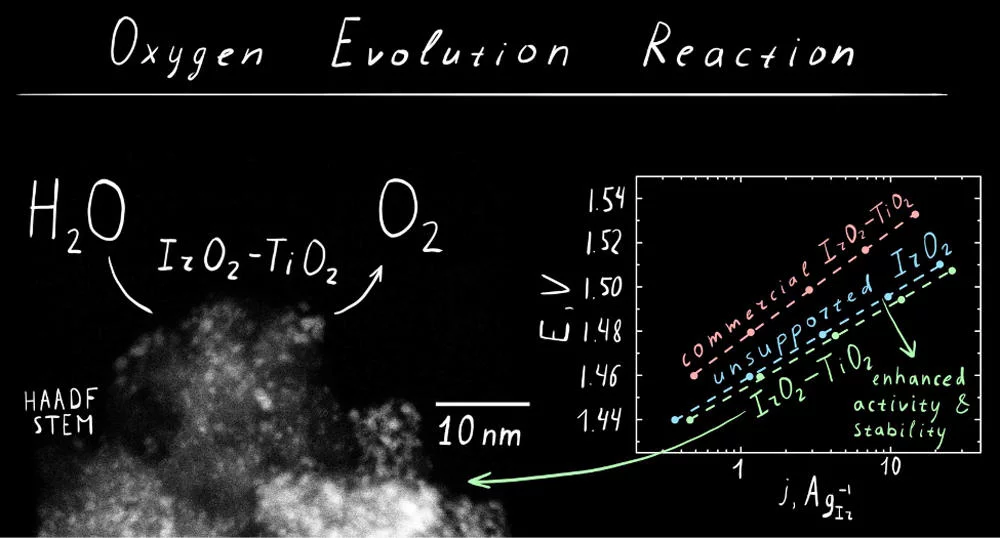
The utilization and development of efficient water electrolyzers for hydrogen production is currently limited due to the sluggish kinetics of the anodic process the oxygen evolution reaction (OER). Moreover, state of the art OER catalysts contain high amounts of expensive and low abundance noble metals such as Ru and Ir, limiting their large scale industrial utilization. Therefore, the development of low cost, highly active, and stable OER catalysts is a key requirement toward the implementation of a hydrogen-based economy. We have developed a synthetic approach to high surface-area chlorine-free iridium oxide nanoparticles dispersed in titania (IrO2-TiO2), which is a highly active and stable OER catalyst in acidic media. IrO2-TiO2 was prepared in one step in molten NaNO3 (Adams fusion method) and consists of ca. 1−2 nm IrO2 particles distributed in a matrix of titania nanoparticles with an overall surface area of 245 m2 g−1. This material contains 40 molM % of iridium and demonstrates improved OER activity and stability in comparison to the commercial benchmark catalyst and state of the art high-surface-area IrO2. Ex situ characterization of the catalyst indicates the presence of iridium hydroxo surface species, which were previously associated with the high OER activity. Operando X-ray absorption studies demonstrate the evolution of the surface species as a function of the applied potential, suggesting the conversion of the initial hydroxo surface layer to the oxo-terminated surface via anodic oxidation (OER regime).
Contact
Dr Maarten NachtegaalSuperXAS beamline
Laboratory for Synchrotron Radiation and Femtochemistry (LSF)
Swiss Light Source, Paul Scherrer Intitute
5232 Villigen-PSI, Switzerland
Telephone: +41 56 310 30 56
E-mail: marten.nachtegaal@psi.ch
Original Publication
IrO2‑TiO2: A High-Surface-Area, Active, and Stable Electrocatalyst for the Oxygen Evolution ReactionEmma Oakton, Dmitry Lebedev, Mauro Povia, Daniel F. Abbott, Emiliana Fabbri, Alexey Fedorov, Maarten Nachtegaal, Christophe Copéret, and Thomas J. Schmidt BR% ACS Catalysis, 17 February 2017
DOI: 10.1021/acscatal.6b03246