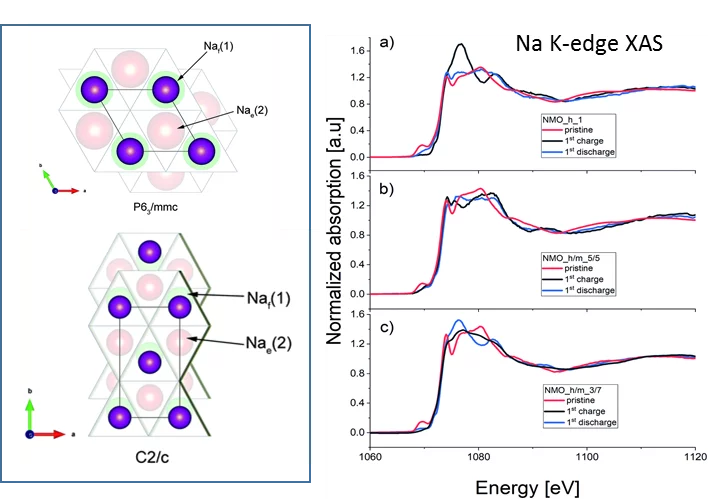
Sodium ion batteries are similar to Li ion batteries, however one of the differences is that the lithium within the cathode materials have been replaced with sodium, which is abundant on Earth. Owing to their high operating potentials and high capacities, cathodes based on sodium transition metal oxides NaxMnyO2 have received recently a great deal of attention. One unanswered question is how Na/Mn local arrangements influence the electrochemical performance of this material. The Na K-edge XAS spectra acquired at the PHOENIX beamline provide a better understanding about the Na atomic positions in the NaxMnyO2 phases appearing during cycling. Measuring the Na absorption spectra in samples with different state of charge, we were able to distinguish between tetrahedral and octahedral coordination of the Na ions in the electrode crystal structure. Consequently, combining these results with operando XRD data we managed to characterize appearing phases and we found that depending of the initial phase composition it is possible to tune the type of high voltage phases. For specific compositions and at high voltage there exists an intergrowth structure between P2 and O2 type phases with intermediate OP4 structure, in which Na ions reside both in tetrahedral and octahedral sites. We then correlated the electrochemical phase evolution of NaxMnyO2 cathodes with its electrochemical properties and established general trends to tailor the materials with high specific charge and good cyclic stability. We also proved that XAS measurements done at PHOENIX beamline are effective tools that help understanding the (de)sodiation mechanism and, as a result, design materials with improved electrochemical properties.
Contact
Energy and Environment Division, OLGA 117
Paul Scherrer Institut
Telephone: +41 56 310 2457
E-mail: petr.novak@psi.ch
Dr. Camelia Borca
Swiss Light Source
Paul Scherrer Institut
Telephone: +41 56 310 5478
Influence of Na/Mn arrangements and P2/P'2 phase ratio on the electrochemical performance of NaxMnO2 cathodes for sodium-ion batteries
A. Kulka, C. Marino, K. Walczak, C. Borca, C. Bolli, P. Novák, and C. Villevieille
Journal of Materials Chemistry A, 2020, 8(12), 6022-6033
DOI: 10.1039/C9TA12176E