Water gets in shape for VUV absorption
Nanometre‑thin, free‑flowing liquid sheets now let Swiss Light Source users record pristine VUV absorption spectra of water, and soon any solvent.
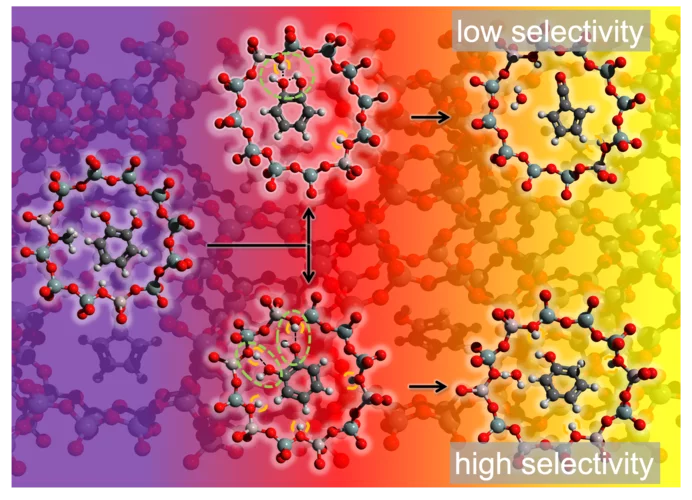
Unveiling the reaction mechanism shines light on the selectivity increase in catalytic processes
Increasing the selectivity of a chemical process through rational catalyst design is the Holy Grail of heterogeneous catalysis. Researchers at PSI and ETH Zürich showcase how revealing hidden steps in reaction pathways can steer processes towards preferred products, as demonstrated in a study focused on biomass valorization.
The Hercules School visits PSI
20 international students visited PSI as part of the renowned Hercules School to learn about our state-of-the-art techniques and methodologies at our large scale facilities.
Comment les «footballènes» se forment dans l’espace
Une équipe internationale de recherche montre comment les fullerènes se forment dans l’espace.
A star is born
Swiss Light Source SLS reveals complex chemistry inside ‘stellar nurseries’
PSI researcher Patrick Hemberger honored in the Rising Stars special issue in Energy & Fuels
To celebrate contributions of highly influential early and mid-career researchers in energy research, the journal Energy & Fuels established an annual recognition of Energy and Fuels Rising Stars.
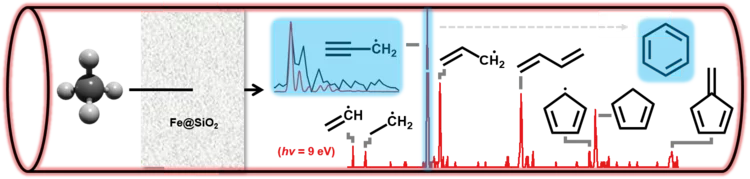
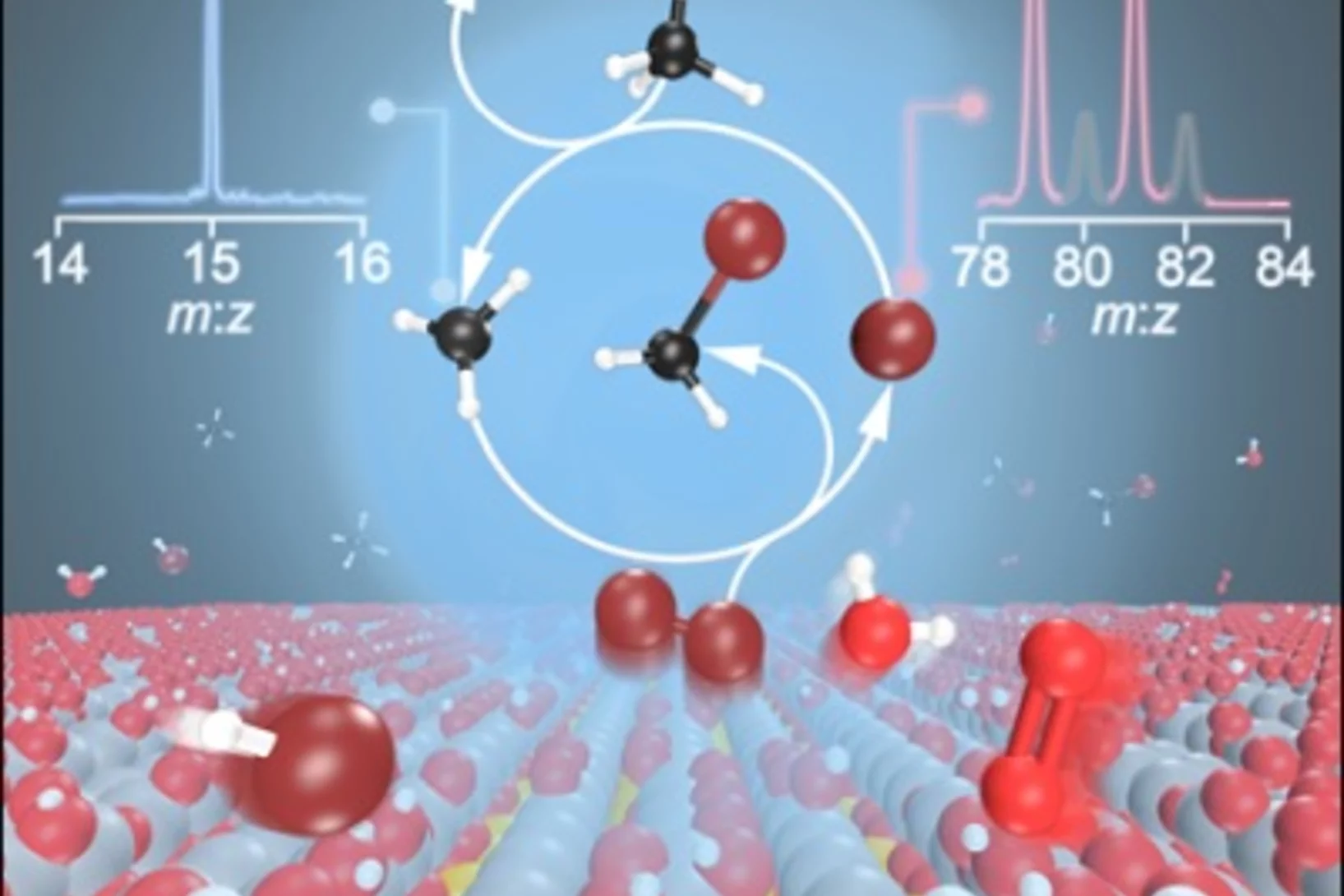
Finding Ketenes in the Methanol to Olefins Process
How are the first olefins formed in the early stages of the methanol-to-olefins process? Detection of two reactive ketene species solves this long-standing puzzle.
Hercules School 2022
PSI hosted again the Hercules School in March 2022. We had the pleasure to welcome 20 international PhD students, PostDocs and scientists to demonstrate our state-of-the-art techniques and methodologies at our large scale facilities, the Swiss Light Source (SLS), the Swiss Spallation Neutron Source (SINQ) and our free electron laser SwissFEL.
Watch them growing: New mechanistic insights into catalytic methane coupling
Methane valorization is a promising technology to utilize this platform compound to produce aromatics and hydrocarbons. Researchers from PSI and ETH Zürich unveiled this reaction mechanism and observed the molecular growth from the ground up. Besides stepwise CH3 addition, novel routes involving the dimerization of resonantly stabilized propargyl (C3H3) radicals to benzene (C6H6) were identified. These mechanistic insights will aid the development of valorisation strategies.
Important elementary reactions of lignin catalytic pyrolysis revealed
To develop sustainable lignin valorization strategies, a solid understanding of the underlying reaction mechanism is critical. By detection of highly reactive and elusive intermediates, new light could be shed on one of the most basic elementary reactions in lignin catalytic fast pyrolysis.
HERCULES SCHOOL 2021 AT PSI
During the week of March 15 – 19, we had the pleasure to welcome 20 international PhD students, PostDocs and assistant professors at PSI, taking part in the first virtual HERCULES SCHOOL on Neutrons & Synchrotron Radiation.
Ruzicka Prize
The Ružička Prize 2020 goes to Dr. Patrick Hemberger (PSI) for his research on understanding the mechanisms of catalytic fast pyrolysis by unveiling reactive intermediates in heterogeneous catalysts.
Methylbismuth: First observation of an organometallic biradical reactive intermediate
Open shell organometallic bismuth are promising agents for catalytic applications, but difficult to characterize due to their high reactivity. The simplest methylbismuth (Bi-CH3), a biradical species, was in-situ synthesized and spectroscopically characterized for the first time. Electronic and thermochemical properties could be obtained, which will guide future synthetic applications.
Selective Alkane Functionalization to Olefins
Light alkanes are abundantly available and cheap resources that are often burned at oil wells because of the missing infrastructure for valorization. Novel technologies are needed for their selective functionalization to use natural gas as an energy vector in the transition between the oil and the renewables era. Catalytic oxyhalogenation may unlock the transformation of cheap and abundant alkanes into commodities. When chlorine-based reactions are compared with bromine, improved selectivities above an iron catalyst arise from surface-confinement of the reaction mechanism in the case of chlorine as halogen.
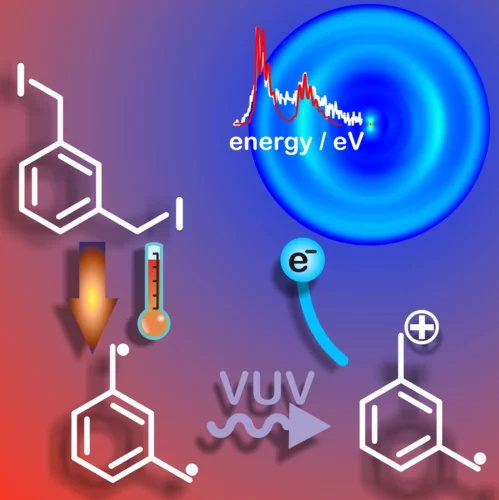
How the methyl group position influences the ultrafast deactivation in aromatic radicals
The resonantly stabilized xylyl radicals (C8H9•) distinctively influence the combustion chemistry and, therefore, ultimately determine the performance of combustion engines. At that, the three different isomers (methyl group in ortho, para or meta position) exhibit notable differences at elevated temperatures. We have tracked down these dynamics on a femtosecond timescale by monitoring the response to preparation of a well-defined electronic and vibrational state.
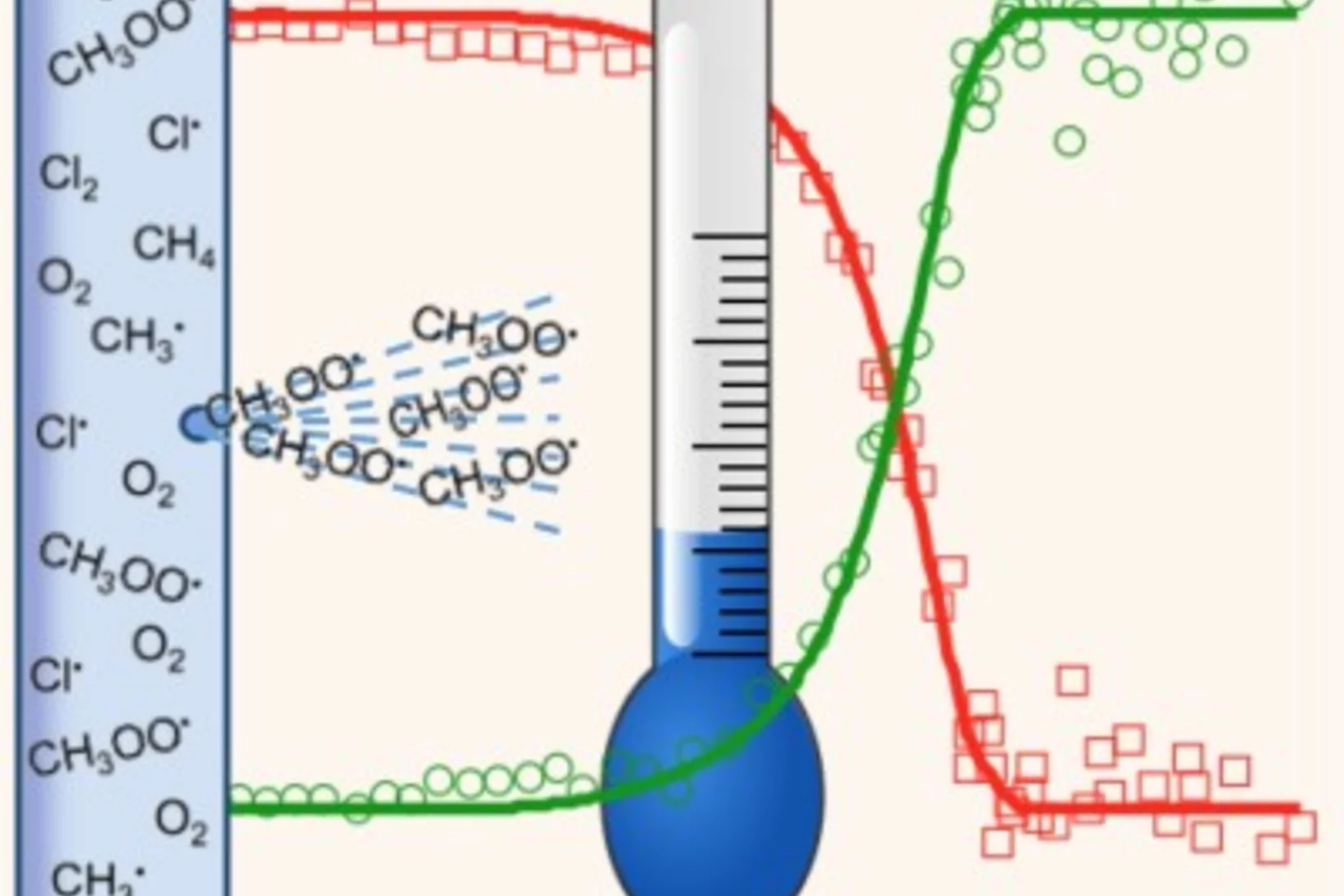
Radical Thermometers and Energetics
Methylperoxy radicals are crucial oxidation intermediates and could be synthesized photolytically in an exothermic reaction. Despite their vanishingly small concentration, their temperature could be measured after a few ten thousand collisions inside the reactor, which opens up the possibility of time-resolved operando temperature measurements. Also the reaction energy to yield methyl cations and oxygen could be determined with sub-kJ mol–1 precision, which firmly anchors the methylperoxy energetics to that of well-known stable species and opens up the possibility of highly accurate radical thermochemistry measurements.
Taming Reactive Molecular Magnets
Studying organic molecular magnets is a challenge, because the high-spin diradical character of these compounds dramatically increases the reactivity and reduces the lifetime. Researchers from PSI, ETH Zurich, Wollongong and Melbourne, Australia succeeded in taming the meta-xylylene diradical and were able to study its electronic and thermochemical properties.
Tracking down radicals in methane oxybromination
Catalytic oxybromination may turn the cheap and abundant feedstock methane into the platform compounds bromomethane and dibromomethane. Yet researchers have been puzzled by the catalysis mechanism, which was speculated to involve free radical intermediates. Operando photoelectron photoion coincidence helped distinguish surface and gas-phase reaction steps and elucidated the crucial halogen-mediated C–H bond activation step, which is driven by elusive bromine and methyl radicals.
Understanding the reaction mechanism in lignin catalytic fast pyrolysis
Lignin is a major constituent of plants, and may be used as a precursor for fuels and fine chemicals. Catalytic fast pyrolysis of lignin is one of the most promising approaches. By using vacuum ultraviolet synchrotron radiation and threshold photoelectron spectroscopy we could identify elusive intermediates, which are responsible for the formation of phenol and benzene and could thus tackle this reaction mechanism. Mechanistic understanding could enable targeted improvement of production methods in the future, beyond the currently used "cook-and-look" approach.
Isomer-Selective Generation and Spectroscopic Characterization of Biofuel Intermediates
Online combustion analysis relies heavily on spectral data to detect reactive intermediates isomer-selectively to establish e.g. kinetic flame models. Due to the difficulty to generate these species cleanly, spectral data are rather scarce. Here we report on the selective generation of three picolyl radical isomers (C5H4N-CH2*) by deamination of aminomethylpyridines. Picolyl radicals are relevant in biofuel combustion, and could now be characterized by threshold photoelectron spectroscopy using synchrotron radiation. Vibrationally resolved bands and distinct ionization energies allow for isomer-specific detection of these elusive species in complex environments and permit us to explore new avenues in soot- and NOx formation kinetics.
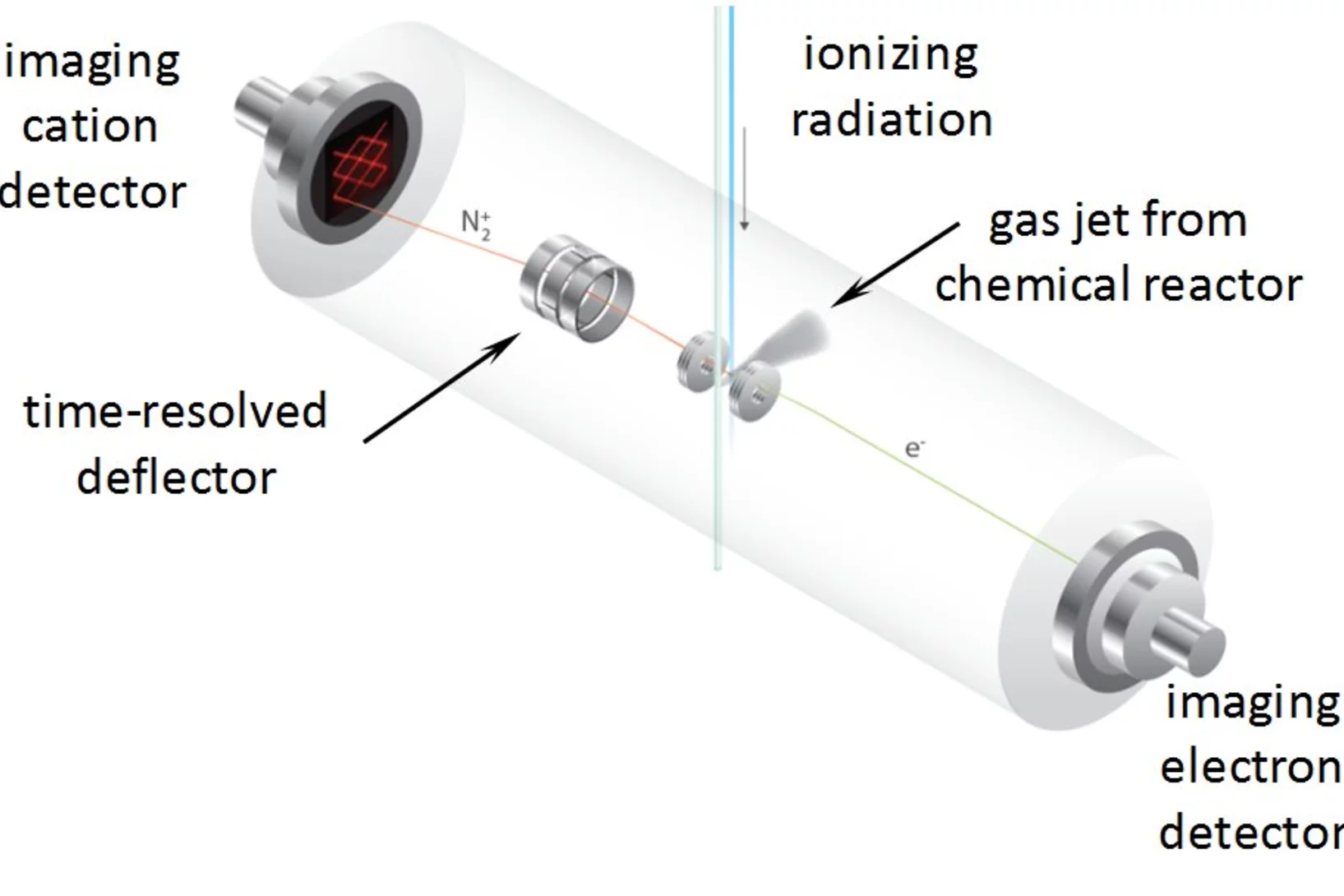
Breaking Through the False Coincidence Barrier in Electron–Ion Coincidence Experiments
The false coincidence background has so far limited the analytical application of PEPICO, photoelectron photoion coincidence. A new photoioin rastering technique has been developed to separate the wheat from the chaff and identify true coincidences based on the ion hit time and position. This expands the dynamic range of the experiment by at least two orders of magnitude, allowing for novel applications to look for reactive intermediates and short lived species in reaction environments.
Controlling tunnelling in methane loss from acetone ions by deuteration
At the imaging Photoelectron Photoion Coincidence (iPEPICO) endstation of the VUV beamline evidence of H-atom tunneling was shown.