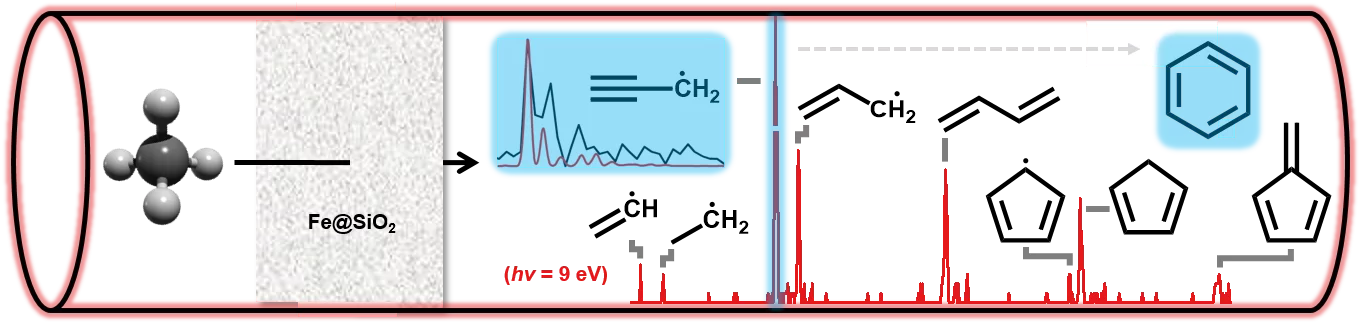
Chemicals, polymers and fuels are essentially chains or rings of carbon and hydrogen with different length and branching. Methane, on the other hand, just consists of four hydrogens and a single carbon atom. Methane activation to produce chemicals and fuels is one of the most-sought after goals. Such conversions are difficult, because of the relative stability of the methane molecule. Through enhanced understanding of the reaction mechanisms, new and better catalysts can be developed. Catalysis over iron-modified silica particles enables activation of methane molecules and allows fusing them together in a stepwise growing process to yield aromatics such as benzene and other hydrocarbons.
Understanding the reaction dynamics on a molecular level is not a straightforward task, will however aid the design of novel catalysts to optimise this process. Scientists from PSI and ETH Zurich utilized mass spectrometric and photoelectron spectroscopic tools available at the VUV (x04db) beamline of the Swiss Light Source to pinpoint early stage intermediates of the chain growth, such as methyl (CH3), ethyl (C2H5), vinyl (C2H3), allyl (C3H5) and propargyl radicals (C3H3).
It was shown, that despite the stepwise (+CH3) growth process also other novel reaction pathways are found, which provide compelling evidence for the selective production of benzene. In particular the resonantly stabilized propargyl radicals (C3H3) can reach large concentrations due to their high stability and mark a direct pathway to form the first aromatic ring, benzene:
2 C3H3 --> C6H6
Despite this pathway, the long-standing collaboration between the Photon Science Devision (PSD) and the Energy and Environment Research Division (ENE) at PSI found that the hydrogen abstraction acetylene addition (HACA) mechanism plays a fundamental role to produce naphthalene from elusive phenyl radicals:
C6H5 + 2 C2H2 --> H + C10H8
These insights are much-needed to optimize the methane valorization process by targeted catalyst design to reach high selectivities towards the desired products.
Original Publication
Direct Evidence on the Mechanism of Methane Conversion under Non-oxidative Conditions over Iron-modified Silica: The Role of Propargyl Radicals Unveiled
Allen Puente-Urbina, Zeyou Pan, Vladimir Paunović, Petr Šot, Patrick Hemberger, Jeroen A. van Bokhoven
Angew. Chem. , 2021.
https://doi.org/10.1002/anie.202107553
Contact
Dr. Patrick Hemberger
Reaction Dynamics Group
Paul Scherrer Institut
Telephone: +41 56 310 3236
E-mail: patrick.hemberger@psi.ch
https://www.psi.ch/lsf/patrick-hemberger
Prof. Dr. Jeroen A. van Bokhoven
Laboratory Leader LSK
Paul Scherrer Institut
Telephone: +41 56 310 5046
E-mail: jeroen.vanbokhoven@psi.ch
and
Professor for Heterogeneous Catalysis
ETH Zurich
Telephone: +41 44 632 5542
E-mail: jeroen.vanbokhoven@chem.ethz.ch
