Curiosity drives my research in the laboratory to gain a fundamental understanding of ice and snow chemistry. This line of research is timely as it tackles key environmental issues, such as winter haze in highly populated regions in Asia and the Arctic. The focus lies on the interfacial physical chemistry of ice and on the fate and chemistry of nanometer-sized aerosol deposits in snow. Next to the environmental relevance, the understudied chemistry in the cold shows fascinating deviations from our textbook knowledge that address fundamental chemistry topics of general interest, such as interfacial acid-base chemistry, chemical systems with low liquid water content, and hydrogen bonding of water molecules.
International working group CATCH
The CATCH mission is to facilitate atmospheric chemistry research within the international community, with a focus on natural processes specific to cold regions of Earth. Cold regions include areas which are seasonally or permanently covered by snow and ice, from the high mountains to the polar ice sheets and sea ice zones as well as regions where ice clouds that undergo chemistry are found. https://www.catchscience.org
- 2022 University of California Los Angeles (UCLA), Sabbatical
- 2015 - present Paul Scherrer Institut PSI Senior Scientist
- 2006 - 2015 Paul Scherrer Institut PSI Scientist
- 2006 parental leave
- 2004 - 2006 Neue Zürcher Zeitung freelance science writer
- 2004 - 2005 University of Toronto PostDoc
- 2004 parental leave
- 1999-2003 Paul Scherrer Institut PSI graduate student
- 1999 - 2003 Universität Bern, Dr. phil.-nat.
- 1995 - 1999 ETH Zürich, Dipl. Chem. ETH
- 1996 - 1997 Norwegian University of Science and Technology Trondheim (NTNU), ETH stipendiary
- 1993 - 1995 Julius-Maximilians-Universität Würzburg, Vordiplom
- Steering committee member of the Physics and Chemistry of Ice (PCI)
- Co-chair of Cryosphere and Atmospheric Chemistry (CATCH)
- Juan Felipe Flórez Ospina, PostDoc
- Luca Longetti, PostDoc
- Yanisha Manoharan, Ph.D. student at ETH Zürich
- Jérôme Gabathuler, Ph.D. student at ETH Zürich, 2018-2022
- Xiangrui Kong, PostDoc, 2015-2017
- Jacinta Edebeli, Ph.D. student at ETH Zürich, 2015-2019
- Astrid Waldner, Ph.D. student at ETH Zürich, 2014-2017
- Thomas Ulrich, Ph.D. student at University of Bern, 2009-2013
-
Bartels-Rausch T, Kong X, Orlando F, Artiglia L, Waldner A, Huthwelker T, et al.
Interfacial supercooling and the precipitation of hydrohalite in frozen NaCl solutions as seen by X-ray absorption spectroscopy
Cryosphere. 2021; 15(4): 2001-2020. https://doi.org/10.5194/tc-15-2001-2021
DORA PSI -
Edebeli J, Trachsel JC, Avak SE, Ammann M, Schneebeli M, Eichler A, et al.
Snow heterogeneous reactivity of bromide with ozone lost during snow metamorphism
Atmospheric Chemistry and Physics. 2020; 20(21): 13443-13454. https://doi.org/10.5194/acp-20-13443-2020
DORA PSI -
Bartels-Rausch T, Jacobi H-W, Kahan TF, Thomas JL, Thomson ES, Abbatt JPD, et al.
A review of air-ice chemical and physical interactions (AICI): liquids, quasi-liquids, and solids in snow
Atmospheric Chemistry and Physics. 2014; 14(3): 1587-1633. https://doi.org/10.5194/acp-14-1587-2014
DORA PSI -
Thomas JL, Stutz J, Frey MM, Bartels-Rausch T, Altieri K, Baladima F, et al.
Fostering multidisciplinary research on interactions between chemistry, biology, and physics within the coupled cryosphere-atmosphere system
Elementa: Science of the Anthropocene. 2019; 7(1): 58 (16 pp.). https://doi.org/10.1525/elementa.396
DORA PSI -
Bartels-Rausch T
Ten things we need to know about ice and snow
Nature. 2013; 494(7435): 27-29. https://doi.org/10.1038/494027a
DORA PSI
-
Chen S, Abouhaidar R, Artiglia L, Yang H, Boucly A, Iezzi L, et al.
Influence of surfactants with differently charged headgroups on the surface propensity of bromide
Journal of Physical Chemistry A. 2025; 129(13): 3085-3097. https://doi.org/10.1021/acs.jpca.4c07539
DORA PSI -
Richter C, Gholami S, Manoharan Y, Buttersack T, Longetti L, Artiglia L, et al.
Uptake of ammonia by ice surfaces at atmospheric temperatures
Faraday Discussions. 2025. https://doi.org/10.1039/d4fd00169a
DORA PSI -
Ammann M, Alpert PA, Artiglia L, Bao F, Bartels-Rausch T, Ospina JFF, et al.
Multiphase chemistry in the atmosphere
Chimia. 2024; 78(11): 754-761. https://doi.org/10.2533/chimia.2024.754
DORA PSI -
Fauré N, Chen J, Artiglia L, Ammann M, Bartels-Rausch T, Kanji ZA, et al.
Formation of sodium chloride on the surface of sulfate-rich gobi desert salt in response to water adsorption
ACS ES&T Air. 2024; 1(11): 1373-1382. https://doi.org/10.1021/acsestair.4c00092
DORA PSI -
Kuhn J, Stutz J, Bartels-Rausch T, Thomas JL, Cesler-Maloney M, Simpson WR, et al.
The interplay between snow and polluted air masses in cold urban environments
Faraday Discussions. 2024. https://doi.org/10.1039/d4fd00176a
DORA PSI -
Bartels-Rausch T, Ammann M
It is time to introduce the next generation of chemists to FAIR and open science
Chimia. 2023; 77(10): 694-696. https://doi.org/10.2533/chimia.2023.694
DORA PSI -
Bartels-Rausch T, Gabathuler JP, Yang H, Manoharan Y, Artiglia L, Ammann M
Removing gas-phase features in near ambient pressure partial Auger-Meitner electron yield oxygen K-edge NEXAFS spectra
Journal of Electron Spectroscopy and Related Phenomena. 2023; 264: 147320 (7 pp.). https://doi.org/10.1016/j.elspec.2023.147320
DORA PSI -
Fauré N, Chen J, Artiglia L, Ammann M, Bartels-Rausch T, Li J, et al.
Unexpected behavior of chloride and sulfate ions upon surface solvation of Martian salt analogue
ACS Earth and Space Chemistry. 2023; 7(2): 350-359. https://doi.org/10.1021/acsearthspacechem.2c00204
DORA PSI -
Hong AC, Ulrich T, Thomson ES, Trachsel J, Riche F, Murphy JG, et al.
Uptake of hydrogen peroxide from the gas phase to grain boundaries: a source in snow and ice
Environmental Science and Technology. 2023; 57(31): 11626-11633. https://doi.org/10.1021/acs.est.3c01457
DORA PSI -
Kong X, Gladich I, Fauré N, Thomson ES, Chen J, Artiglia L, et al.
Adsorbed water promotes chemically active environments on the surface of sodium chloride
Journal of Physical Chemistry Letters. 2023; 14(26): 6151-6156. https://doi.org/10.1021/acs.jpclett.3c00980
DORA PSI -
Mallet MD, Humphries RS, Fiddes SL, Alexander SP, Altieri K, Angot H, et al.
Untangling the influence of Antarctic and Southern Ocean life on clouds
Elementa: Science of the Anthropocene. 2023; 11(1): 00130 (18 pp.). https://doi.org/10.1525/elementa.2022.00130
DORA PSI -
Bartels-Rausch T, Kong X, Orlando F, Artiglia L, Waldner A, Huthwelker T, et al.
Interfacial supercooling and the precipitation of hydrohalite in frozen NaCl solutions as seen by X-ray absorption spectroscopy
Cryosphere. 2021; 15(4): 2001-2020. https://doi.org/10.5194/tc-15-2001-2021
DORA PSI -
Yang H, Boucly A, Gabathuler JP, Bartels-Rausch T, Artiglia L, Ammann M
Ordered hydrogen bonding structure of water molecules adsorbed on silver iodide particles under subsaturated conditions
Journal of Physical Chemistry C. 2021; 125(21): 11628-11635. https://doi.org/10.1021/acs.jpcc.1c01767
DORA PSI -
Edebeli J, Trachsel JC, Avak SE, Ammann M, Schneebeli M, Eichler A, et al.
Snow heterogeneous reactivity of bromide with ozone lost during snow metamorphism
Atmospheric Chemistry and Physics. 2020; 20(21): 13443-13454. https://doi.org/10.5194/acp-20-13443-2020
DORA PSI -
Kong X, Castarède D, Boucly A, Artiglia L, Ammann M, Bartels-Rausch T, et al.
Reversibly physisorbed and chemisorbed water on carboxylic salt surfaces under atmospheric conditions
Journal of Physical Chemistry C. 2020; 124(9): 5263-5269. https://doi.org/10.1021/acs.jpcc.0c00319
DORA PSI -
Avak SE, Trachsel JC, Edebeli J, Brütsch S, Bartels‐Rausch T, Schneebeli M, et al.
Melt‐induced fractionation of major ions and trace elements in an Alpine snowpack
Journal of Geophysical Research: Earth Surface. 2019; 124(7): 1647-1657. https://doi.org/10.1029/2019JF005026
DORA PSI -
Bartels-Rausch T, Montagnat M
The physics and chemistry of ice
Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2019; 377(2146): 20190138 (5 pp.). https://doi.org/10.1098/rsta.2019.0138
DORA PSI -
Edebeli J, Ammann M, Bartels-Rausch T
Microphysics of the aqueous bulk counters the water activity driven rate acceleration of bromide oxidation by ozone from 289–245 K
Environmental Science: Processes and Impacts. 2019; 21(1): 63-73. https://doi.org/10.1039/C8EM00417J
DORA PSI -
Orlando F, Artiglia L, Yang H, Kong X, Roy K, Waldner A, et al.
Disordered adsorbed water layers on TiO2 nanoparticles under subsaturated humidity conditions at 235 K
Journal of Physical Chemistry Letters. 2019; 10(23): 7433-7438. https://doi.org/10.1021/acs.jpclett.9b02779
DORA PSI -
Thomas JL, Stutz J, Frey MM, Bartels-Rausch T, Altieri K, Baladima F, et al.
Fostering multidisciplinary research on interactions between chemistry, biology, and physics within the coupled cryosphere-atmosphere system
Elementa: Science of the Anthropocene. 2019; 7(1): 58 (16 pp.). https://doi.org/10.1525/elementa.396
DORA PSI -
Trachsel JC, Avak SE, Edebeli J, Schneebeli M, Bartels-Rausch T, Bruetsch S, et al.
Microscale rearrangement of ammonium induced by snow metamorphism
Frontiers in Earth Science. 2019; 7: 194 (19 pp.). https://doi.org/10.3389/feart.2019.00194
DORA PSI -
Ammann M, Artiglia L, Bartels-Rausch T
X-Ray excited electron spectroscopy to study gase-liquid interfaces of atmospheric relevance
In: Faust JA, House JE, eds. Physical chemistry of gas-liquid interfaces. Developments in physical & theoretical chemistry. Amsterdam: Elsevier; 2018:135-166. https://doi.org/10.1016/B978-0-12-813641-6.00006-6
DORA PSI -
Corral Arroyo P, Bartels-Rausch T, Alpert PA, Dumas S, Perrier S, George C, et al.
Particle-phase photosensitized radical production and aerosol aging
Environmental Science and Technology. 2018; 52(14): 7680-7688. https://doi.org/10.1021/acs.est.8b00329
DORA PSI -
Kong X, Wolf MJ, Roesch M, Thomson ES, Bartels-Rausch T, Alpert PA, et al.
A continuous flow diffusion chamber study of sea salt particles acting as cloud nuclei: deliquescence and ice nucleation
Tellus, Series B: Chemical and Physical Meteorology. 2018; 70(1): 1463806 (11 pp.). https://doi.org/10.1080/16000889.2018.1463806
DORA PSI -
Waldner A, Artiglia L, Kong X, Orlando F, Huthwelker T, Ammann M, et al.
Pre-melting and the adsorption of formic acid at the air-ice interface at 253 K as seen by NEXAFS and XPS
Physical Chemistry Chemical Physics. 2018; 20(37): 24408-24417. https://doi.org/10.1039/C8CP03621G
DORA PSI -
Artiglia L, Edebeli J, Orlando F, Chen S, Lee M-T, Corral Arroyo P, et al.
A surface-stabilized ozonide triggers bromide oxidation at the aqueous solution-vapour interface
Nature Communications. 2017; 8(1): 700 (8 pp.). https://doi.org/10.1038/s41467-017-00823-x
DORA PSI -
Barsotti F, Bartels-Rausch T, De Laurentiis E, Ammann M, Brigante M, Mailhot G, et al.
Photochemical formation of nitrite and nitrous acid (HONO) upon irradiation of nitrophenols in aqueous solution and in viscous secondary organic aerosol proxy
Environmental Science and Technology. 2017; 51(13): 7486-7495. https://doi.org/10.1021/acs.est.7b01397
DORA PSI -
Bartels-Rausch T, Orlando F, Kong X, Artiglia L, Ammann M
Experimental evidence for the formation of solvation shells by soluble species at a nonuniform air-ice interface
ACS Earth and Space Chemistry. 2017; 1(9): 572-579. https://doi.org/10.1021/acsearthspacechem.7b00077
DORA PSI -
Gržinić G, Bartels-Rausch T, Türler A, Ammann M
Efficient bulk mass accommodation and dissociation of N2O5 in neutral aqueous aerosol
Atmospheric Chemistry and Physics. 2017; 17(10): 6493-6502. https://doi.org/10.5194/acp-17-6493-2017
DORA PSI -
Kong X, Waldner A, Orlando F, Artiglia L, Huthwelker T, Ammann M, et al.
Coexistence of physisorbed and solvated HCl at warm ice surfaces
Journal of Physical Chemistry Letters. 2017; 8(19): 4757-4762. https://doi.org/10.1021/acs.jpclett.7b01573
DORA PSI -
Meusel H, Elshorbany Y, Kuhn U, Bartels-Rausch T, Reinmuth-Selzle K, Kampf CJ, et al.
Light-induced protein nitration and degradation with HONO emission
Atmospheric Chemistry and Physics. 2017; 17(19): 11819-11833. https://doi.org/10.5194/acp-17-11819-2017
DORA PSI -
González Palacios L, Corral Arroyo P, Aregahegn KZ, Steimer SS, Bartels-Rausch T, Nozière B, et al.
Heterogeneous photochemistry of imidazole-2-carboxaldehyde: HO2 radical formation and aerosol growth
Atmospheric Chemistry and Physics. 2016; 16(18): 11823-11836. https://doi.org/10.5194/acp-16-11823-2016
DORA PSI -
Orlando F, Waldner A, Bartels-Rausch T, Birrer M, Kato S, Lee M-T, et al.
The environmental photochemistry of oxide surfaces and the nature of frozen salt solutions: a new in situ XPS approach
Topics in Catalysis. 2016; 59(5-7): 591-604. https://doi.org/10.1007/s11244-015-0515-5
DORA PSI -
Gržinić G, Bartels-Rausch T, Berkemeier T, Türler A, Ammann M
Viscosity controls humidity dependence of N2O5 uptake to citric acid aerosol
Atmospheric Chemistry and Physics. 2015; 15(23): 13615-13625. https://doi.org/10.5194/acp-15-13615-2015
DORA PSI -
Bartels-Rausch T, Jacobi H-W, Kahan TF, Thomas JL, Thomson ES, Abbatt JPD, et al.
A review of air-ice chemical and physical interactions (AICI): liquids, quasi-liquids, and solids in snow
Atmospheric Chemistry and Physics. 2014; 14(3): 1587-1633. https://doi.org/10.5194/acp-14-1587-2014
DORA PSI -
George C, D'Anna B, Herrmann H, Weller C, Vaida V, Donaldson DJ, et al.
Emerging areas in atmospheric photochemistry
In: McNeill VF, Ariya PA, eds. Atmospheric and aerosol chemistry. Topics in current chemistry. Berlin Heidelberg: Srpinger; 2014:1-53. https://doi.org/10.1007/128_2012_393
DORA PSI -
Gržinić G, Bartels-Rausch T, Birrer M, Türler A, Ammann M
Production and use of 13N labeled N2O5 to determine gas-aerosol interaction kinetics
Radiochimica Acta. 2014; 102(11): 1025-1034. https://doi.org/10.1515/ract-2014-2244
DORA PSI -
Legrand M, Preunkert S, Frey M, Bartels-Rausch T, Kukui A, King MD, et al.
Large mixing ratios of atmospheric nitrous acid (HONO) at Concordia (East Antarctic Plateau) in summer: a strong source from surface snow?
Atmospheric Chemistry and Physics. 2014; 14(18): 9963-9976. https://doi.org/10.5194/acp-14-9963-2014
DORA PSI -
Bartels-Rausch T, Wren SN, Schreiber S, Riche F, Schneebeli M, Ammann M
Diffusion of volatile organics through porous snow: impact of surface adsorption and grain boundaries
Atmospheric Chemistry and Physics. 2013; 13(14): 6727-6739. https://doi.org/10.5194/acp-13-6727-2013
DORA PSI -
Bartels-Rausch T
Ten things we need to know about ice and snow
Nature. 2013; 494(7435): 27-29. https://doi.org/10.1038/494027a
DORA PSI -
Křepelová A, Bartels-Rausch T, Brown MA, Bluhm H, Ammann M
Adsorption of acetic acid on ice studied by ambient-pressure XPS and partial-electron-yield NEXAFS spectroscopy at 230-240 K
Journal of Physical Chemistry A. 2013; 117(2): 401-409. https://doi.org/10.1021/jp3102332
DORA PSI -
Bartels-Rausch T, Schneebeli M
Comment on 'Possible contribution of triboelectricity to snow-air interactions'
Environmental Chemistry. 2012; 9(2): 119-120. https://doi.org/10.1071/EN11147
DORA PSI -
Bartels-Rausch T, Bergeron V, Cartwright JHE, Escribano R, Finney JL, Grothe H, et al.
Ice structures, patterns, and processes: a view across the icefields
Reviews of Modern Physics. 2012; 84(2): 885-944. https://doi.org/10.1103/RevModPhys.84.885
DORA PSI -
Donaldson DJ, Ammann M, Bartels-Rausch T, Pöschl U
Standard states and thermochemical kinetics in heterogeneous atmospheric chemistry
Journal of Physical Chemistry A. 2012; 116(24): 6312-6316. https://doi.org/10.1021/jp212015g
DORA PSI -
McNeill VF, Grannas AM, Abbatt JPD, Ammann M, Ariya P, Bartels-Rausch T, et al.
Organics in environmental ices: sources, chemistry, and impacts
Atmospheric Chemistry and Physics. 2012; 12(20): 9653-9678. https://doi.org/10.5194/acp-12-9653-2012
DORA PSI -
Riche F, Bartels-Rausch T, Schreiber S, Ammann M, Schneebeli M
Temporal evolution of surface and grain boundary area in artificial ice beads and implications for snow chemistry
Journal of Glaciology. 2012; 58(210): 815-817. https://doi.org/10.3189/2012JoG12J058
DORA PSI -
Ulrich T, Ammann M, Leutwyler S, Bartels-Rausch T
The adsorption of peroxynitric acid on ice between 230 K and 253 K
Atmospheric Chemistry and Physics. 2012; 12(4): 1833-1845. https://doi.org/10.5194/acp-12-1833-2012
DORA PSI -
Bartels-Rausch T, Ulrich T, Huthwelker T, Ammann M
A novel synthesis of the N-13 labeled atmospheric trace gas peroxynitric acid
Radiochimica Acta. 2011; 99(5): 285-292. https://doi.org/10.1524/ract.2011.1830
DORA PSI -
Bartels-Rausch T, Krysztofiak G, Bernhard A, Schläppi M, Schwikowski M, Ammann M
Photoinduced reduction of divalent mercury in ice by organic matter
Chemosphere. 2011; 82(2): 199-203. https://doi.org/10.1016/j.chemosphere.2010.10.020
DORA PSI -
Sosedova Y, Rouvière A, Bartels-Rausch T, Ammann M
UVA/Vis-induced nitrous acid formation on polyphenolic films exposed to gaseous NO2
Photochemical and Photobiological Sciences. 2011; 10(10): 1680-1690. https://doi.org/10.1039/c1pp05113j
DORA PSI -
Bartels-Rausch T, Brigante M, Elshorbany YF, Ammann M, D'Anna B, George C, et al.
Humic acid in ice: photo-enhanced conversion of nitrogen dioxide into nitrous acid
Atmospheric Environment. 2010; 44(40): 5443-5450. https://doi.org/10.1016/j.atmosenv.2009.12.025
DORA PSI -
Kerbrat M, Huthwelker T, Bartels-Rausch T, Gäggeler HW, Ammann M
Co-adsorption of acetic acid and nitrous acid on ice
Physical Chemistry Chemical Physics. 2010; 12(26): 7194-7202. https://doi.org/10.1039/b924782c
DORA PSI -
Bartels-Rausch T, Huthwelker T, Jöri M, Gäggeler H, Ammann M
Interaction of gaseous elemental mercury with snow surfaces: laboratory investigation
Environmental Research Letters. 2008; 3(4): 045009 (5 pp.). https://doi.org/10.1088/1748-9326/3/4/045009
DORA PSI -
Clifford D, Bartels-Rausch T, Donaldson DJ
Suppression of aqueous surface hydrolysis by monolayers of short chain organic amphiphiles
Physical Chemistry Chemical Physics. 2007; 9(11): 1362-1369. https://doi.org/10.1039/b617079j
DORA PSI -
Bartels-Rausch T, Huthwelker T, Gäggeler HW, Ammann M
Atmospheric pressure coated-wall flow-tube study of acetone adsorption on ice
Journal of Physical Chemistry A. 2005; 109(20): 4531-4539. https://doi.org/10.1021/jp045187l
DORA PSI -
Bartels-Rausch T, Guimbaud C, Gäggeler HW, Ammann M
The partitioning of acetone to different types of ice and snow between 198 and 223 K
Geophysical Research Letters. 2004; 31(16): L16110 (4 pp.). https://doi.org/10.1029/2004GL020070
DORA PSI -
Guimbaud C, Bartels-Rausch T, Ammann M
An atmospheric pressure chemical ionization mass spectrometer (APCI-MS) combined with a chromatographic technique to measure the adsorption enthalpy of acetone on ice
International Journal of Mass Spectrometry. 2003; 226(2): 279-290. https://doi.org/10.1016/S1387-3806(03)00019-8
DORA PSI -
Bartels-Rausch T, Eichler B, Zimmermann P, Gäggeler HW, Ammann M
The adsorption enthalpy of nitrogen oxides on crystalline ice
Atmospheric Chemistry and Physics. 2002; 2(3): 235-247. https://doi.org/10.5194/acp-2-235-2002
DORA PSI
Research: The chemical properties of interfacial water
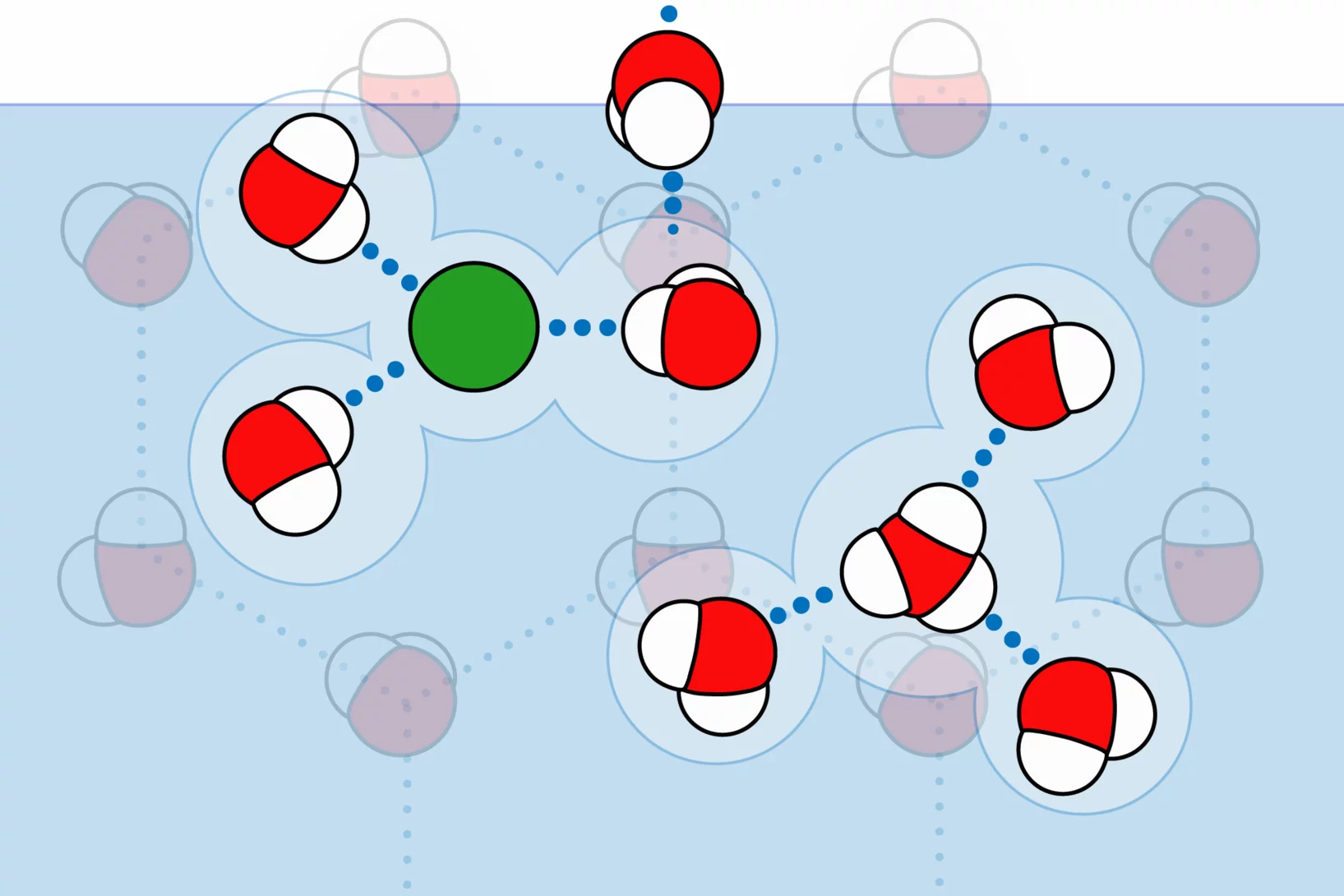
This line of research deals with the characterization of the hydrogen-bonding network structure of adsorbed water at the surface of environmentally relevant mineral surfaces by Near Ambient Pressure X-ray excited electron spectroscopy at the Swiss Light Source of PSI (SLS). The studies revealed liquid-like features of adsorbed water at sub-freezing temperatures with an impact on the ability to act as ice nuclei and to drive atmospheric chemistry. A unique feature of the SLS endstation is the precise temperature (80°C to -80°C) and water vapor pressure (up to 6 mbar) regulation allowing to probe any condition in the phase diagram of aerosol and ice.
Current Funding
Selected Publications
-
Yang H, Boucly A, Gabathuler JP, Bartels-Rausch T, Artiglia L, Ammann M
Ordered hydrogen bonding structure of water molecules adsorbed on silver iodide particles under subsaturated conditions
Journal of Physical Chemistry C. 2021; 125(21): 11628-11635. https://doi.org/10.1021/acs.jpcc.1c01767
DORA PSI -
Orlando F, Artiglia L, Yang H, Kong X, Roy K, Waldner A, et al.
Disordered adsorbed water layers on TiO2 nanoparticles under subsaturated humidity conditions at 235 K
Journal of Physical Chemistry Letters. 2019; 10(23): 7433-7438. https://doi.org/10.1021/acs.jpclett.9b02779
DORA PSI
Research: Molecular level view on the air-ice interface
Taking full advantage of surface-sensitive X-ray absorption spectroscopy and X-ray photoemission spectroscopy at near ambient pressure, this research has provided direct experimental evidence on how acidic trace gases, such as HCl, modify the hydrogen bonding network at the ice surface. The evolving picture is that acidic trace gases form liquid-like solvation shells in an elsewise ice-like interfacial region upon adsorption. This finding raises open questions on the impact of photochemistry and heterogeneous chemistry at the interfacial region of ice and snow in the environment, given the importance of multiphase chemistry of snow and ice clouds for Earth’s geochemical cycles and climate system.
Current Funding
Selected Publications
-
Bartels-Rausch T, Kong X, Orlando F, Artiglia L, Waldner A, Huthwelker T, et al.
Interfacial supercooling and the precipitation of hydrohalite in frozen NaCl solutions as seen by X-ray absorption spectroscopy
Cryosphere. 2021; 15(4): 2001-2020. https://doi.org/10.5194/tc-15-2001-2021
DORA PSI -
Křepelová A, Bartels-Rausch T, Brown MA, Bluhm H, Ammann M
Adsorption of acetic acid on ice studied by ambient-pressure XPS and partial-electron-yield NEXAFS spectroscopy at 230-240 K
Journal of Physical Chemistry A. 2013; 117(2): 401-409. https://doi.org/10.1021/jp3102332
DORA PSI -
Bartels-Rausch T, Orlando F, Kong X, Artiglia L, Ammann M
Experimental evidence for the formation of solvation shells by soluble species at a nonuniform air-ice interface
ACS Earth and Space Chemistry. 2017; 1(9): 572-579. https://doi.org/10.1021/acsearthspacechem.7b00077
DORA PSI -
Kong X, Waldner A, Orlando F, Artiglia L, Huthwelker T, Ammann M, et al.
Coexistence of physisorbed and solvated HCl at warm ice surfaces
Journal of Physical Chemistry Letters. 2017; 8(19): 4757-4762. https://doi.org/10.1021/acs.jpclett.7b01573
DORA PSI
Research: Multiphase chemistry in the cold
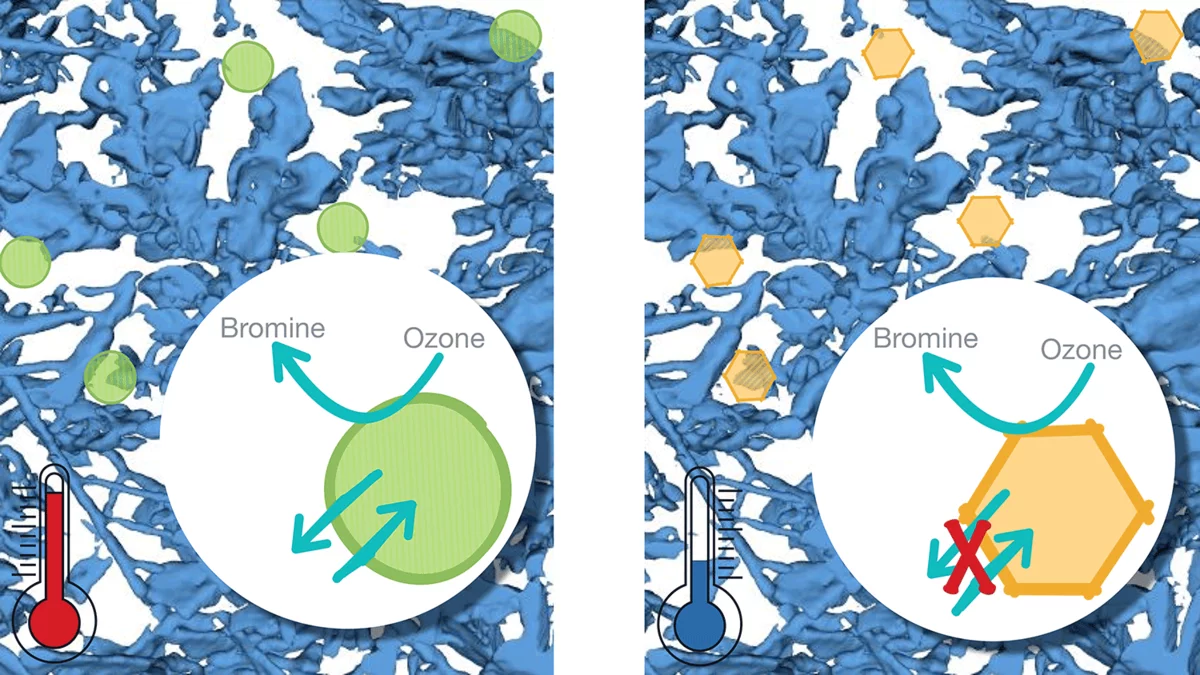
Research over the past decades has provided an impressive observational basis for large scale effects of multiphase chemistry in snow, such as substantial modification of the composition and of the chemical reactivity of the lowermost atmosphere in polar regions and the ability of toxins to enter the marine food web. This fundamental laboratory based research investigates the chemical reactivity of solutes and reactants embedded at different locations in snow. Questions tackled are the impact of complex composition, of microphysical properties, and of phase changes on the reactivity. In cooperation with the Schneebeli group in Davos, we further investigated how snow metamorphism influences multiphase chemistry at snow surfaces.
Selected Publications
-
Edebeli J, Trachsel JC, Avak SE, Ammann M, Schneebeli M, Eichler A, et al.
Snow heterogeneous reactivity of bromide with ozone lost during snow metamorphism
Atmospheric Chemistry and Physics. 2020; 20(21): 13443-13454. https://doi.org/10.5194/acp-20-13443-2020
DORA PSI -
Edebeli J, Ammann M, Bartels-Rausch T
Microphysics of the aqueous bulk counters the water activity driven rate acceleration of bromide oxidation by ozone from 289–245 K
Environmental Science: Processes and Impacts. 2019; 21(1): 63-73. https://doi.org/10.1039/C8EM00417J
DORA PSI -
Artiglia L, Edebeli J, Orlando F, Chen S, Lee M-T, Corral Arroyo P, et al.
A surface-stabilized ozonide triggers bromide oxidation at the aqueous solution-vapour interface
Nature Communications. 2017; 8(1): 700 (8 pp.). https://doi.org/10.1038/s41467-017-00823-x
DORA PSI
Development: Under the hood projects
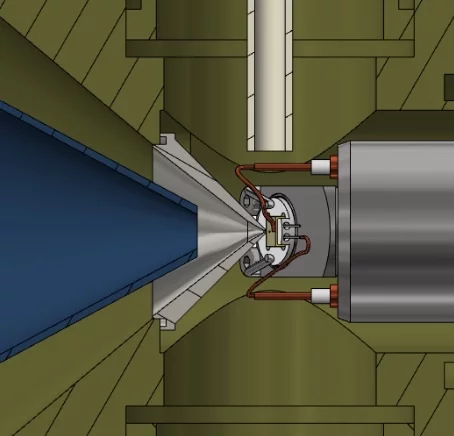
I contribute to developing X-ray excited electron spectroscopy for atmospheric research at the ISS endstation at the Swiss Light Source. The approach is to bring our established know-how in dosing and detecting atmospheric trace gases to this Near Ambient Pressure Photoemission facility. This development work also includes going further in open and fair data management by establishing FAIR data processing routines and workflows for the above research and by introducing ETH's data management system openBIS to PSI.
Current Funding
Selected Publications
-
Bartels-Rausch T, Ammann M
It is time to introduce the next generation of chemists to FAIR and open science
Chimia. 2023; 77(10): 694-696. https://doi.org/10.2533/chimia.2023.694
DORA PSI -
Ammann M, Artiglia L, Bartels-Rausch T
X-Ray excited electron spectroscopy to study gase-liquid interfaces of atmospheric relevance
In: Faust JA, House JE, eds. Physical chemistry of gas-liquid interfaces. Developments in physical & theoretical chemistry. Amsterdam: Elsevier; 2018:135-166. https://doi.org/10.1016/B978-0-12-813641-6.00006-6
DORA PSI -
Orlando F, Waldner A, Bartels-Rausch T, Birrer M, Kato S, Lee M-T, et al.
The environmental photochemistry of oxide surfaces and the nature of frozen salt solutions: a new in situ XPS approach
Topics in Catalysis. 2016; 59(5-7): 591-604. https://doi.org/10.1007/s11244-015-0515-5
DORA PSI