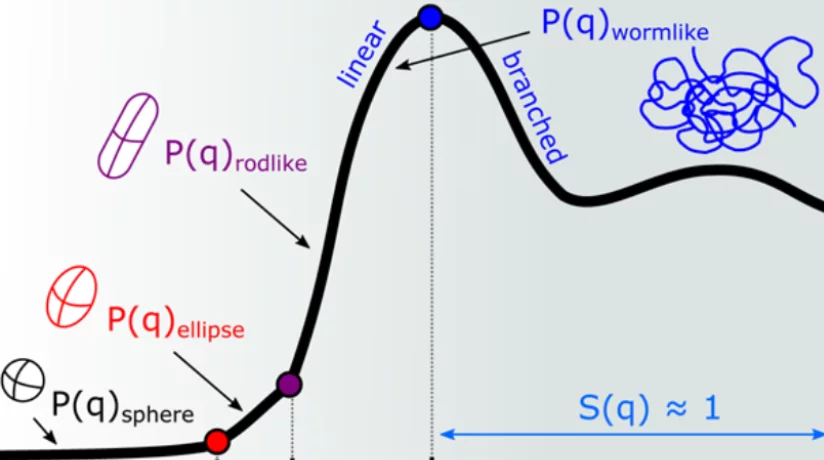
In ionic surfactant micelles, basic interactions among distinct parts of surfactant monomers, their counterion, and additives are fundamental to tuning molecular self-assembly and enhancing viscoelasticity. Here, we investigate the addition of sodium salicylate (NaSal) to hexadecyltrimethylammonium chloride and bromide (CTAC and CTAB) and 1-hexadecylpyridinium chloride and bromide (CPyCl and CPyBr), which have distinct counterions and headgroup structures but the same hydrophobic tail. Different contrasts are obtained from small-angle neutron scattering (SANS), which probes differences between the nucleus of atoms, and X-rays SAXS, which probes differences in electron density. If combined, this contrast allows us to define specific intramicellar length scales and intermicellar interactions. SANS signals are sensitive to the contrast between the solvent (D2O) and the hydrocarbonic tails in the micellar core (hydrogen), and SAXS can access the inner structure of the polar shell because the headgroups, counterions, and penetrated salt have higher electron densities compared to the solvent and to the micellar core. The number density, intermicellar distances, aggregation number, and inter/intramicellar repulsions are discussed on the basis of the dependence of the structure factor and form factor on the micellar aggregate morphology. Therefore, we confirm that micellar growth can be tuned by variations in the flexibility and size of the the headgroup as well as the ionic dissociation rate of its counterion. Additionally, we show that the counterion binding is even more significant to the development of viscoelasticity than the headgroup structure of a surfactant molecule. This is a surprising finding, showing the importance of electrostatic charges in the self-assembly process of ionic surfactant molecules.
Reference: V. Lutz-Bueno et al, Langmuir 33, 2617 (2017)
Read full article: here