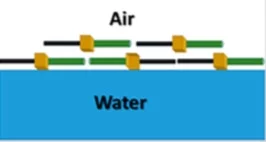
Semifluorinated alkanes form monolayers with interesting properties at the air–water interface due to their pronounced amphi-solvophobic nature and the stiffness of the fluorocarbons. In the present work, using a combination of structural and dynamic probes, we investigated how small molecular changes can be used to control the properties of such an interface, in particular its organization, rheology, and reversibility during compression–expansion cycles. Starting from a reference system perfluor(dodecyl)dodecane, we first retained the linear structure but changed the linkage groups between the alkyl chains and the fluorocarbons, by introducing either a phenyl group or two oxygens. Next, the molecular structure was changed from linear to branched, with four side chains (two fluorocarbons and two hydrocarbons) connected to extended aromatic cores. Neutron reflectivity at the air–water interface and scanning force microscopy on deposited films show how the changes in the molecular structure affect molecular arrangement relative to the interface. Rheological and compression–expansion measurements demonstrate the significant consequences of these changes in molecular structure and interactions on the interfacial properties. Remarkably, even with these simple molecules, a wide range of surface rheological behaviors can be engineered, from viscous over viscoelastic to brittle solids, for very similar values of the surface pressure.
- About the CenterschliessenAbout the Center
- Our Research
- Facilities
- SINQ: Swiss Spallation Neutron Source
- SμS: Swiss Muon Source
- CHRISP: Swiss Research Infrastructure for Particle Physics
- Scientific Advisory Committees
- Publications
- Jobs & Education