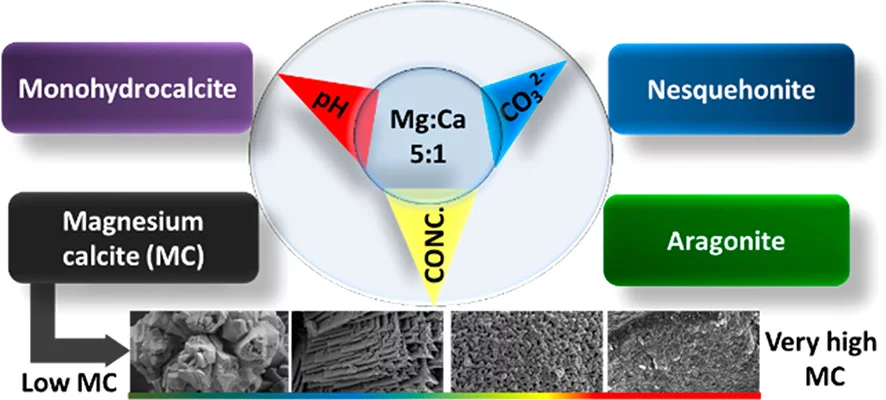
Magnesium rich calcites are important functional biominerals. For example, they can be found in protective shells or eye lenses. Natural organism provide a surprisingly high degree of control on the amount of magnesium incorporation into calcites by yet not well understood mechanisms.
Here we systematically explore the role of magnesium in calcium carbonate polymorph selection in saturated solutions with the goal of better understanding the natural biomineralization processes. A systematic study was performed on the influence of Mg ions on the crystallization of Mg-rich calcites at ambient conditions. Three key parameters of the solution have been varied: pH, carbonate concentration and total effective concentration, while keeping the Mg:Ca ratio at 5:1, approximately the prevailing ratio in most oceans. The results obtained using X-ray diffraction (XRD) and atomic absorption spectroscopy (AAS) enables us to identify the particular conditions favoring the formation of high-magnesium calcite under ambient conditions. Further, through in situ X-ray absorption spectroscopy (XAS) we established that the formation mechanism of magnesium calcium carbonates occurs via the formation of a metastable amorphous phase. These insights open up new opportunities for fabricating biomimetic materials with tunable structures and mechanical properties.
The identification of thermodynamic conditions, where very high magnesium rich calcites (50% Mg/50% Ca) form under ambient conditions of temperature and pressure may also be an important for an old mystery in geochemistry: Very high magnesium rich calcite is believed to be the precursor for dolomite, but despite its frequent occurrence in nature, the pathway for dolomite formation is yet unknown.
Contact
Dr. Thomas Huthwelker
Swiss Light Source
Paul Scherrer Institut
Telephone: +41 56 310 5314
E-mail: thomas.huthwelker@psi.ch
Original Publication
Tuning the Incorporation of Magnesium into Calcite during its Crystallization from Additive-Free Aqueous Solution
Jacinta M. Xto, Huachuan Du, Camelia N. Borca, Ester Amstadt, Jeroen A. van Bokhoven, Thomas Huthwelker
Cryst. Growth Des., 2019, 19, 8, 4385-4394
DOI: 10.1021/acs.cgd.9b00179