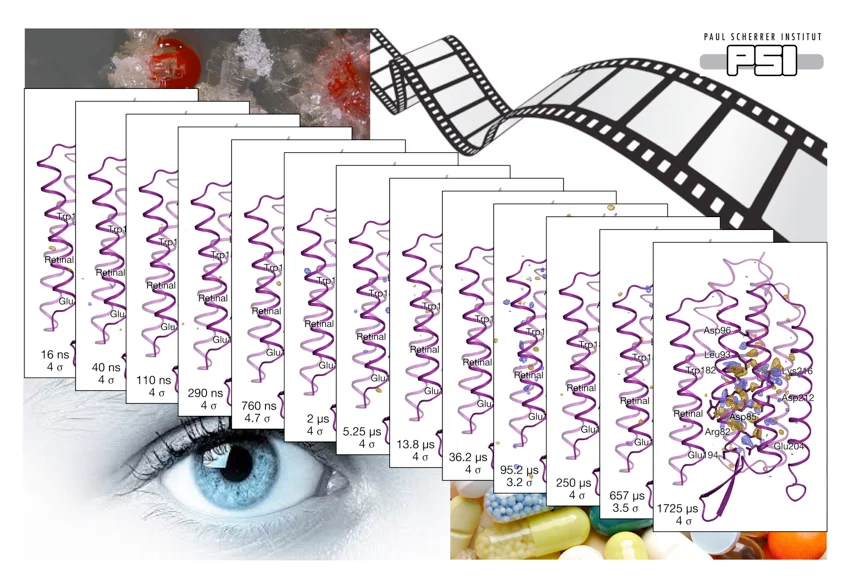
Bacteriorhodopsin (bR) is a light-driven proton pump and a model membrane transport protein. We used time-resolved serial femtosecond crystallography at an x-ray free electron laser to visualize conformational changes in bR from nanoseconds to milliseconds following photoactivation. An initially twisted retinal chromophore displaces a conserved tryptophan residue of transmembrane helix F on the cytoplasmic side of the protein while dislodging a key water molecule on the extracellular side. The resulting cascade of structural changes throughout the protein shows how motions are choreographed as bR transports protons uphill against a transmembrane concentration gradient.
Contact
Dr. Joerg StandfussGroup Leader, LBR, OFLB/007
Paul Scherrer Institut
Telephone: +41 56 310 2586
E-mail: joerg.standfuss@psi.ch
Original Publication
A three-dimensional movie of structural changes in bacteriorhodopsinEriko Nango, Antoine Royant, Minoru Kubo, Takanori Nakane, Cecilia Wickstrand, Tetsunari Kimura, Tomoyuki Tanaka, Kensuke Tono9, Changyong Song, Rie Tanaka, Toshi Arima, Ayumi Yamashita, Jun Kobayashi, Toshiaki Hosaka, Eiichi Mizohata, Przemyslaw Nogly, Michihiro Sugahara, Daewoong Nam, Takashi Nomura, Tatsuro Shimamura, Dohyun Im, Takaaki Fujiwara, Yasuaki Yamanaka, Byeonghyun Jeon, Tomohiro Nishizawa, Kazumasa Oda, Masahiro Fukuda, Rebecka Andersson, Petra Båth, Robert Dods, Jan Davidsson, Shigeru Matsuoka, Satoshi Kawatake, Michio Murata, Osamu Nureki, Shigeki Owada, Takashi Kameshima, Takaki Hatsui, Yasumasa Joti, Gebhard Schertler, Makina Yabashi, Ana-Nicoleta Bondar, Jörg Standfuss, Richard Neutze, So Iwata
23 Dec 2016
DOI: 10.1126/science.aah3497