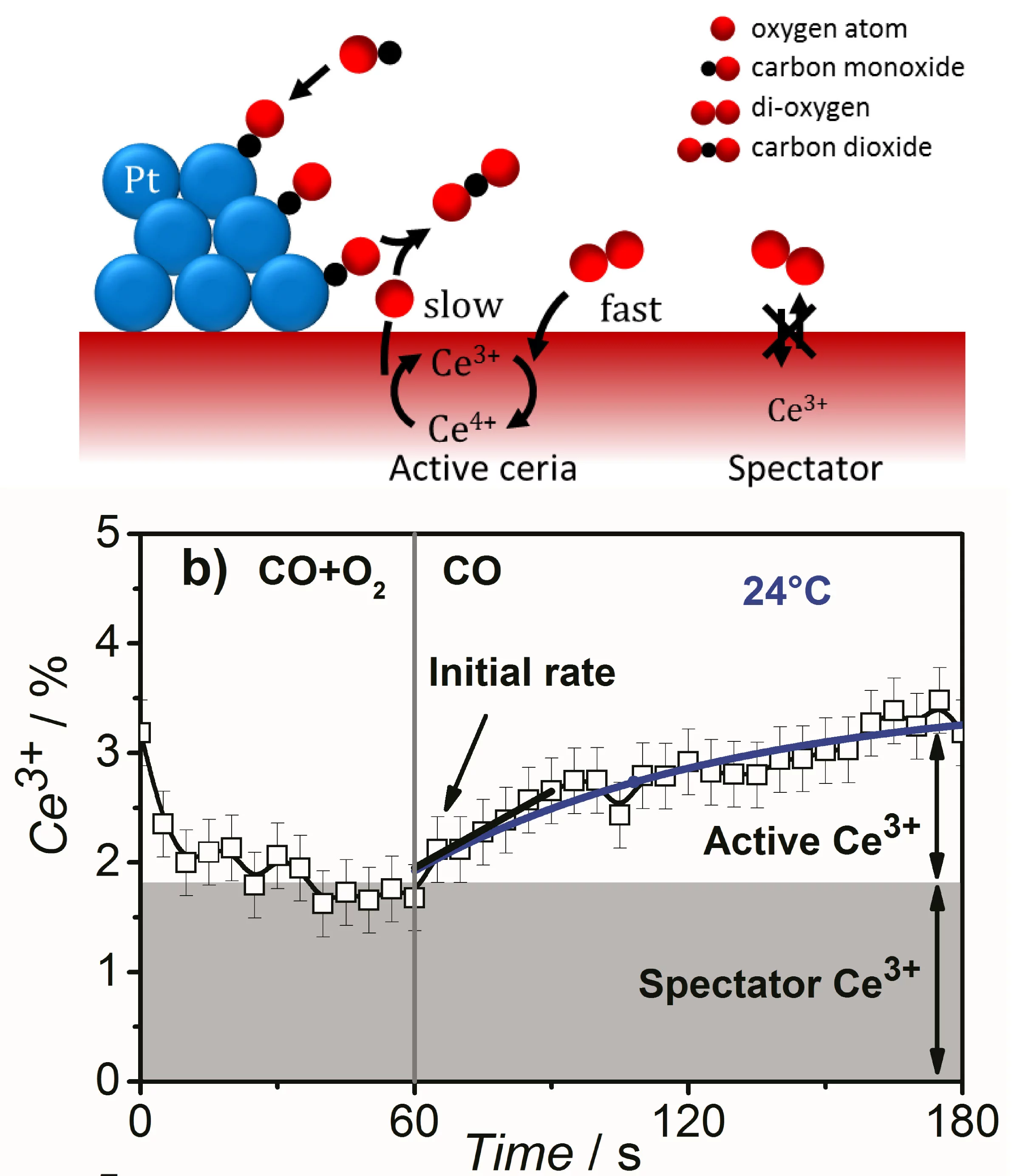
Identification of active species and the rate-determining reaction steps are crucial for optimizing the performance of oxygen-storage materials, which play an important role in catalysts lowering automotive emissions, as electrode materials for fuel cells, and as antioxidants in biomedicine. We demonstrated that active Ce3+ species in a ceria-supported platinum catalyst during CO oxidation are short-lived and therefore cannot be observed under steady-state conditions. Using time-resolved resonant X-ray emission spectroscopy, we quantitatively correlated the initial rate of Ce3+ formation under transient conditions to the overall rate of CO oxidation under steady-state conditions and showed that ceria reduction is a kinetically relevant step in CO oxidation, whereas a fraction of Ce3+ was present as spectators. This approach can be applied to various catalytic processes involving oxygen-storage materials and reducible oxides to distinguish between redox and nonredox catalytic mechanisms.
Original Publication
Catalytically Active and Spectator Ce3+ in Ceria-Supported Metal CatalystsRene Kopelent, Jeroen A. van Bokhoven, Jakub Szlachetko, Jacinta Edebeli, Cristina Paun, Maarten Nachtegaal, and Olga V. Safonova
Angewandte Chemie, May (2015)
DOI: 10.1002/ange.201503022