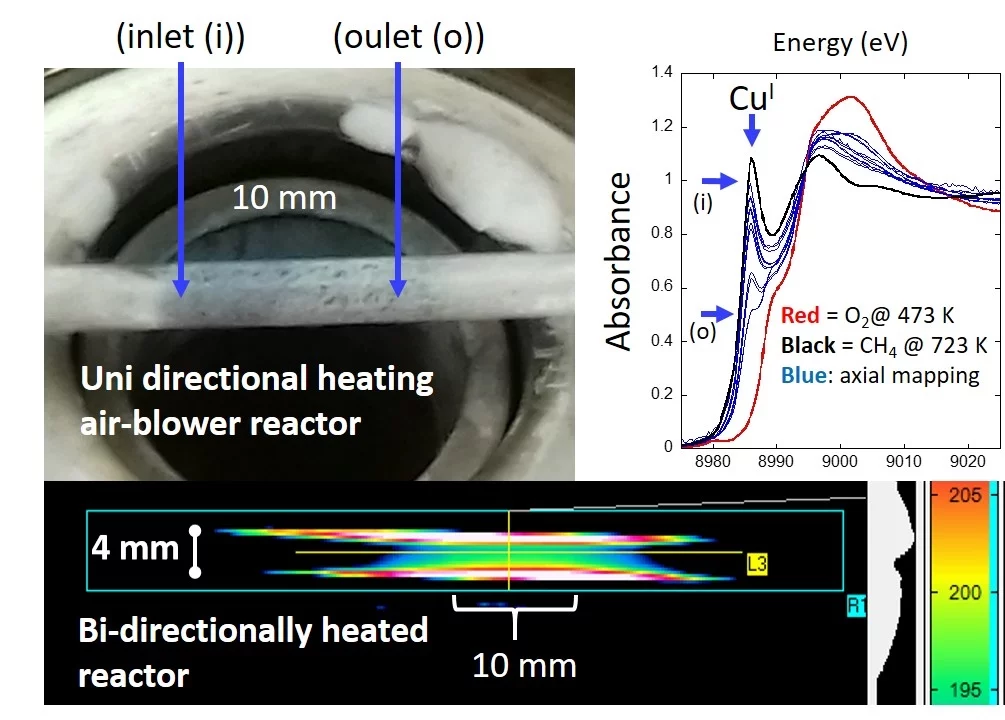
Understanding how structure relates to function in catalysis and numerous other areas, and on that basis designing better materials, is a central goal of much research. Axiomatic to this idea is that any given experiment, or set of experiments, are conducted such that the results obtained truly reflect the nature of the material under study, and are not distorted in any way as a result of the methods used to obtained them. In this study, we have used both reactive chemistry and infrared imaging to assess how well different experimental arrangements, of types that are commonly used, provide one of the foundations upon which reliable study of materials behavior can be achieved; that, in the absence of any reactive chemistry the sample bed is presented in an of isothermal manner. Whether your intention is to measure kinetics, or just to establish more qualitative structure-function relationships, starting from a position where you know that the entirety of your sample is experiencing the same temperature to an acceptable degree, is fundamental. This is especially so if your probe of structure, as is increasingly the case, samples a volume of the sample that is much, much, smaller than the entirety of the sample bed (upon which most commonly applied measures of reactivity, such as mass spectrometry and gas chromatography report). This work shows just how easy it can be to get oneself into a decidedly sub-optimal experimental situation when using some commonly supplied and widely used reactor configurations, but also how to avoid them through different methods of reactor characterization and through the adoption of best practice.
Additional Information
Contact
Dr. Mark A. Newton
PostDoc
ETH Zurich
Telephone: +41 44 633 74 60
E-mail: mark.newton@chem.ethz.ch
Original Publication
On isothermality in some commonly used plug flow reactors for X-ray based investigations of catalysts
DOI: 10.1039/C9CY00464E