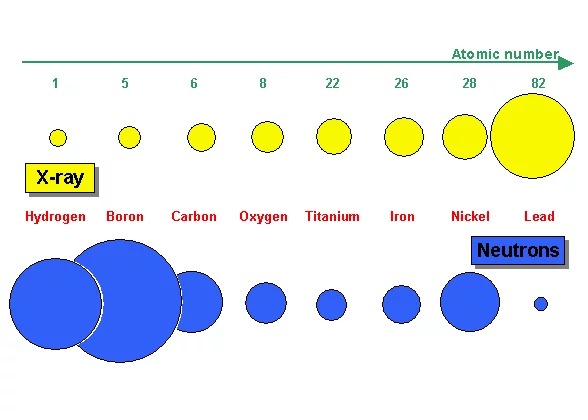
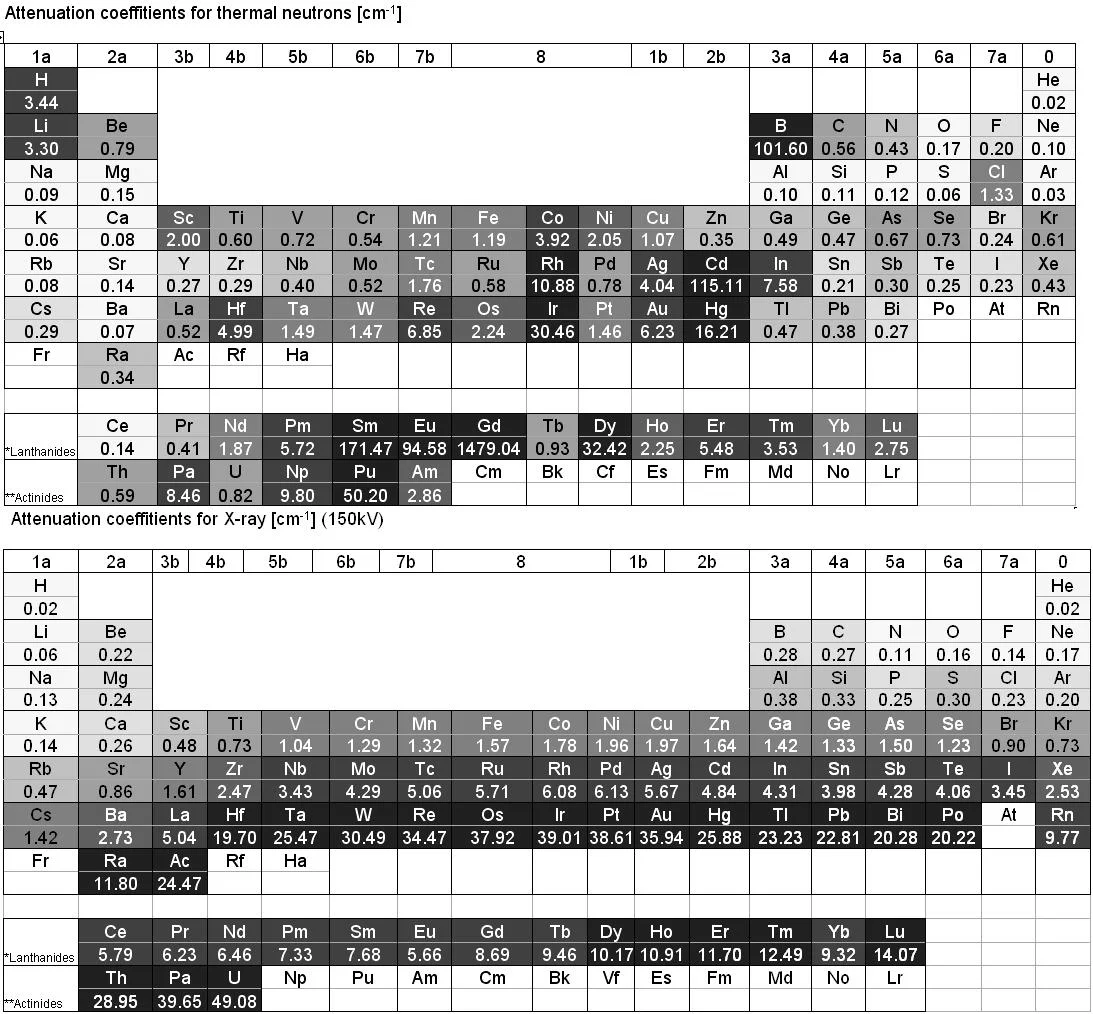
X-ray is electromagnetic radiation which mainly interacts with the electrons of an atom. Thus the more electrons an atom has (i.e. with increasing atomic number Z) the higher the probability for X-ray interaction as shown in Figure 7a. X-ray is therefore an excellent imaging modality for biolological material e.g the human body containing a lot of water. Quite contrary, neutron interaction probability is high in water due to the high scattering probability with the hydrogen atom. Thus a 1 cm thick layer of water is almost opaque for thermal neutrons. The neutron as an electrically neutral particle rather interacts with the atomic nucleus. It is able to transmit thick layers e.g. of metallic material as shown e.g. for lead in Figure 7a and b.
The different radiation matter interaction characteristic is the reason for another effect i.e. activation of nuclei by neutron capture producing instable (mainly short lived) radioactive isotopes. Samples have to be tested for radioactivity after neutron imaging before they can leave the beamline.
For imaging the quite different type of X-ray and neutron source characteristics induce further discrepancies. Due to very high brightness of e.g. synchrotron radiation sources very high spatial resolutions are possible i.e. below 1 µm. For µ-focus X-ray tubes enlarging cone-beam optics are feasible, which allows to get high spatial resolution for small samples. Neutron sources have comparably lower source brightness and extracted neutron beams cannot easily be focussed down to spot sizes below 1 mm. Neutron imaging therefore relies mainly on parallel beam irradiation and effective spatial resolution is often limited by the thickness of the scintillator (some 10 µm's as thinnest layer).