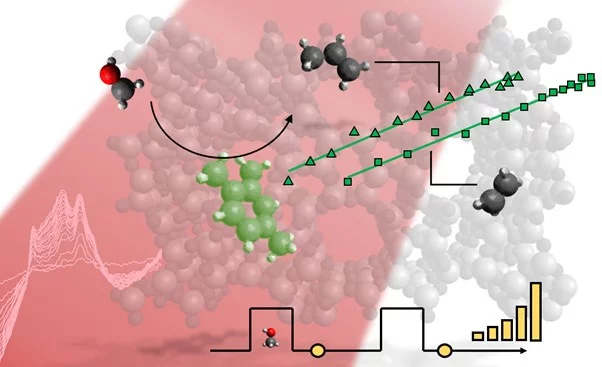
The methanol-to-olefins (MTO) process catalyzed by zeolites is attracting attention as an olefin synthesis route starting from green methanol as an alternative to the fossil fuel industry. To control the selectivity of the process the nature and working principle of the catalytically active site must be understood. In this work, we correlated the selectivity of the process over ZSM-5 (MFI), SSZ-13 (CHA) and ferrierite (FER) zeolite catalysts with the formation of different carbenium ions that stably reside in the porosity. Using an operando transient DRIFTS/GC methodology we could identify polymethylbenzenium ions and alkyl-substituted cyclopentenyl cations over ZSM-5 and we could draw linear relationships between the IR feature of the former and short olefins concentration by GC. Similarly, we identified polymethylbenzenium ion species in SSZ-13 and correlated it with ethene, propene and propane evolution, while on ferrierite allylic carbocations can be possibly related to the formation of C2-C6 olefins with a high selectivity towards C4 and C5 olefins.
The interrelationship between the selectivity in the MTO process, the zeolite topology and the presence of specific carbenium ions was clarified in this work. This information can then guide in the choice and design of zeolite/carbenium ion systems for a specific olefin selectivity.
Read further here.
Contact
Dr Davide Ferri
E-mail: davide.ferri@psi.ch
Luca Maggiulli
E-mail: luca.maggiulli@psi.ch
Prof. J.A. van Bokhoven
E-mail: jeroen.vanbokhoven@psi.ch
Original Publication
Correlating the nature of carbenium ions in zeolites to the product distribution in the methanol-to-olefins process
L. Maggiulli, V.L. Sushkevich, O. Kröcher, J.A. van Bokhoven, D. Ferri, ACS Catal., 14 (2024) 11477
DOI: 10.1021/acscatal.4c03185