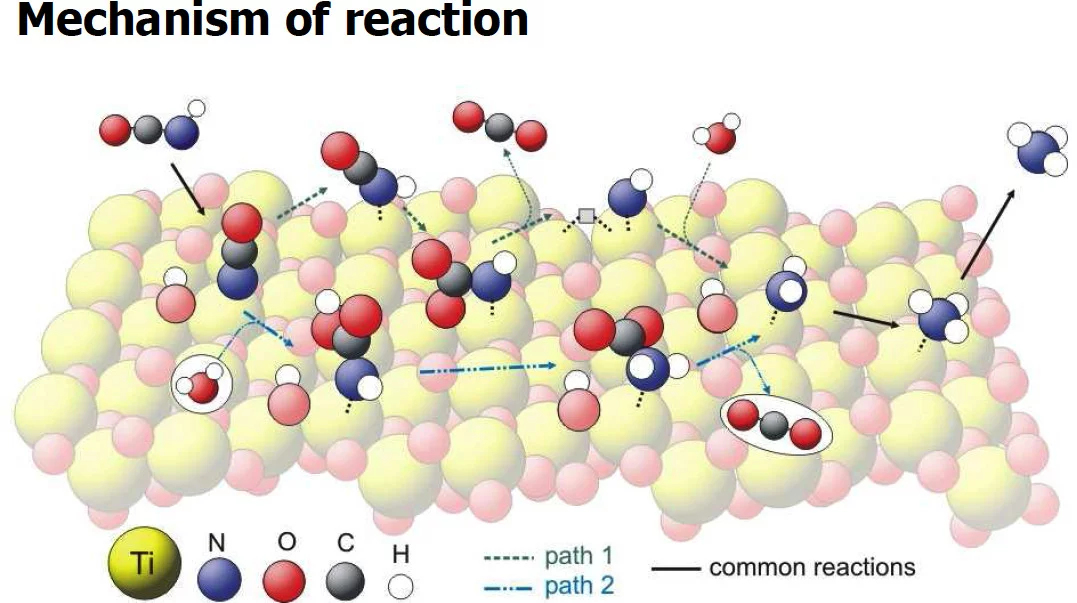
The hydrolysis of isocyanic acid was studied experimentally and theoretically and a reaction mechanism on different catalysts was established. The decreasing NOx emission limits for diesel vehicles impel the further development of the existing NOx deactivation technologies, particularly the selective catalytic reduction (SCR) of nitrogen oxides with urea. In the urea-SCR process, urea is injected into the hot exhaust gas, where it thermally decomposes into isocyanic acid (HNCO) and ammonia. HNCO quickly hydrolyses on the surface of SCR catalysts and even faster on the surface of specialized hydrolysis catalysts. The theoretical Density Functional Theory (DFT) method with cluster model was used. The reaction path was studied by adsorption of water and isocyanic acid, followed by optimization of the adsorbates structure. Additionally, vibrational analyses of the adsorbates were made. In the experimental part activity tests and surface sensitive techniques (XRD, XPS and DRIFTS) were applied. Measured DRIFT spectra could be reproduced by theoretical calculations. Based on the computational screening of different catalysts we can confirm the experimental results, which show that TiO2 is the best catalyst for isocyanic acid hydrolysis. Presented methodology of DFT modeling and "virtual" catalyst screening can be used for successful combination with different experimental methods available at PSI and for variety of questions and complex catalytic systems.