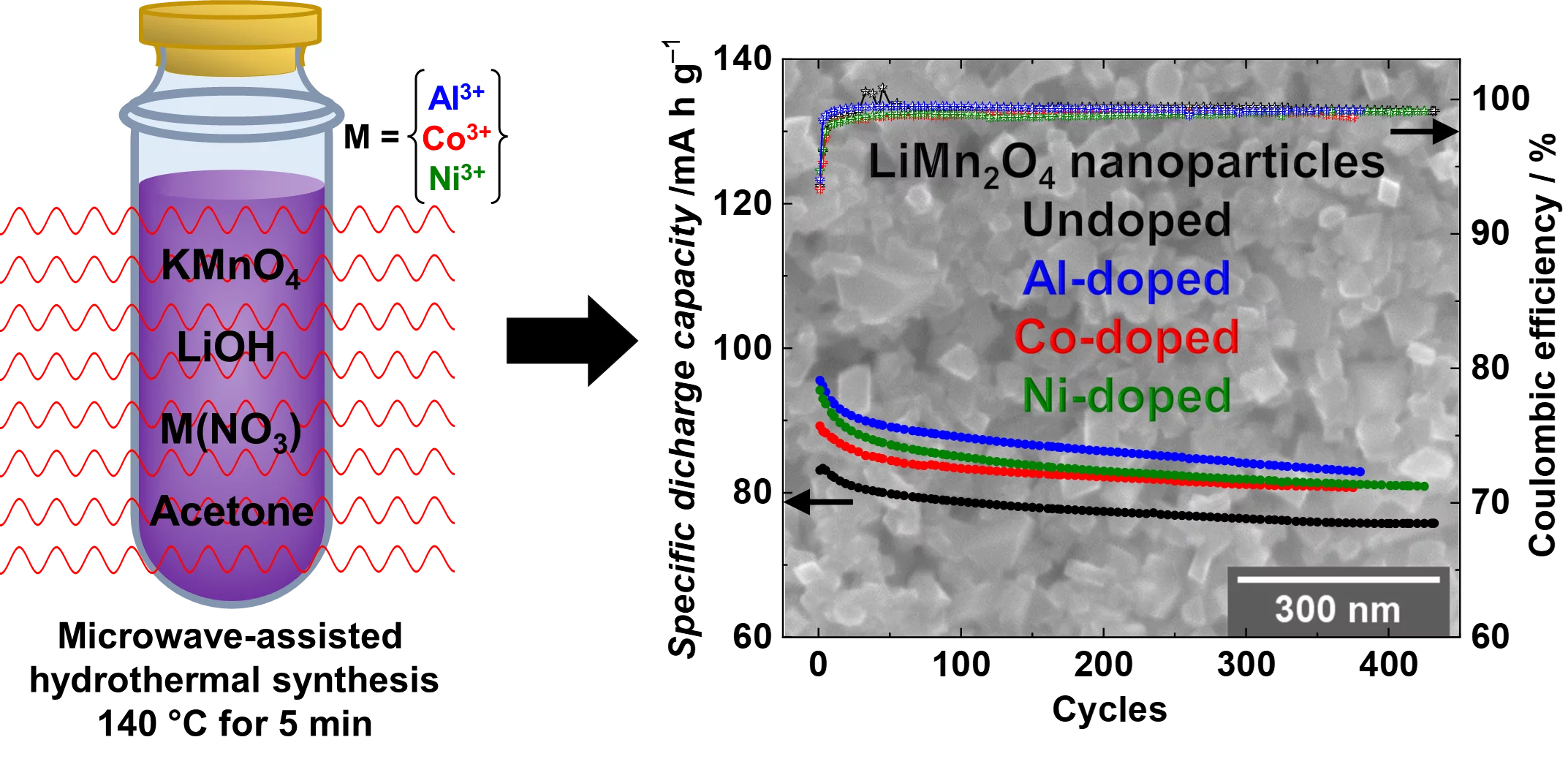
Li-rich nanoparticles of Li1+xMn2-xO4 doped with Al, Co or Ni are successfully synthesized using a facile, fast and efficient microwave-assisted hydrothermal route. In this study, we demonstrate that nanocrystallinity and cationic doping play an important role in improving the electrochemical performance with respect to LiMn2O4 microparticles. They significantly reduce the charge-transfer resistance, lower the 1st cycle irreversible capacity to 6%, and achieve a capacity retention between 85 and 90% after 380 cycles, with excellent columbic efficiency close to 99%.
The most common commercial high voltage cathode materials for lithium-ion batteries (LIB) storage devices are layered oxides based on Ni and Co, frequently denoted as LiNixCoyMnzO2 (NCM) and LiNixCoyAlzO2 (NCA) families. However, due to the high cost of Ni and Co, research on battery materials has changed focus towards the development of new cathode materials, based on safety, low cost and being abundant non-toxic elements. In this context, spinel LiMn2O4 as green and more sustainable batteries materials has shown great potential for LIB applications but still suffer from degradation mechanism during cycling causing an irreversible loss of capacity which are related to (i) the slow dissolution of manganese at the electrode-electrolyte interface and (ii) the strong presence of the irreversible Jahn-Teller distortion in its structure. In order to mitigate the capacity fading and improve the cycling performance of LiMn2O4, we decided in our study to (i) reducing the particle size to the nanoscale; (ii) enrich LiMn2O4 with excess of lithium and (iii) partially substitute (1 to 3%) the Mn3+ by Al, Co or Ni cations. The synthesis of the LiMn2O4 is carried out by a facile, fast and efficient microwave-assisted hydrothermal route at a low temperature of 140 °C for a short time of 5 min from aqueous based-solutions.
SAXS and SEM confirmed the average size of the particles between (60 to 100 nm) and synchrotron X-ray diffraction validated the formation of highly crystalline Li-rich Li1+xMn2-xO4 cubic spinel phase. X-ray absorption spectroscopy analysis at the Co and Ni K- and L-edges verify that the dopants are within the Li1+xMn2-xO4 spinel structure and are inactive during cycling in the bulk and at the surface. From the XANES at Mn K-edge it was possible to monitor the Mn oxidation state in the bulk of the nanoparticles, and it was suggested that the insertion of the Li+ ions is much easier in the nano-sized particles compared to the micron-sized, supported also by the higher columbic efficiency 94% during the 1st cycle. Mn L-edge spectra show that after long cycling the Mn oxidation state in the bulk differs from the one on the surface proposed to be caused by the surface Mn disproportion reaction. The cationic doping helps to mitigate the Mn dissolution with respect to the undoped nano-sized spinels as shown by the ICP measurements.
Contact
Dr. Mario El Kazzi, Group Leader of Battery Materials and Diagnostics
Paul Scherrer Institut
5232 Villigen PSI
Telephone: +41 56 310 51 49
E-mail: mario.el-kazzi@psi.ch
Original Publication
High performance doped Li-rich Li1+xMn2-xO4 cathodes nanoparticles synthesized by facile, fast and efficient microwave-assisted hydrothermal route
Juliana B. Falqueto, Adam H. Clark, Aleš Štefančič, Glen J. Smales, Carlos A. F. Vaz, Albert J. Schuler, Nerilso Bocchi, Mario El Kazzi
ACS Appl. Energy Mater. (2022)
DOI: 10.1021/acsaem.2c00902
Acknowledgements
FAPESP – São Paulo Research Foundation, process no. 2018/16158-6 and 2019/25700-1.