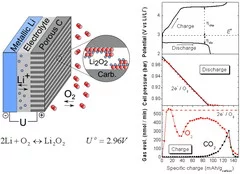
The high-energy rechargeable Li-O2 battery has been subject to intensive research worldwide during the past years. The Li-O2 cell mainly comprises a negative (e.g. Li metal) and positive (e.g. porous carbon) electrode separated by an electronically insulating, but Li+ conducting electrolyte layer. In order to study the cell chemistry, a differential electrochemical mass spectrometry setup based on a set of valves, a pressure sensor and a quadrupole mass spectrometer has been developed. On galvanostatic discharge, oxygen dissolves in the non-aqueous electrolyte, reduces at the porous carbon surface to form mainly Li2O2, as determined from the linear decrease in the oxygen pressure corresponding to a ratio of 2e- per O2 consumed. On charge, the discharge product is oxidized, the lithium ions return to the negative electrode and oxygen gas evolves. Although the oxygen evolution rate initially reaches 2e-/O2, it rapidly drops as the cell over-potential increases. In addition, the evolution of CO2 at 4.3 V vs Li+/Li clearly demonstrates the existence of parasitic side reactions. The D-DEMS, as successfully developed at PSI, is a key tool for analyzing the O2 gas usage, without which conclusions on the cell rechargeability can hardly be drawn.