Simulations on "Piz Daint" explain surprising mineral behaviour
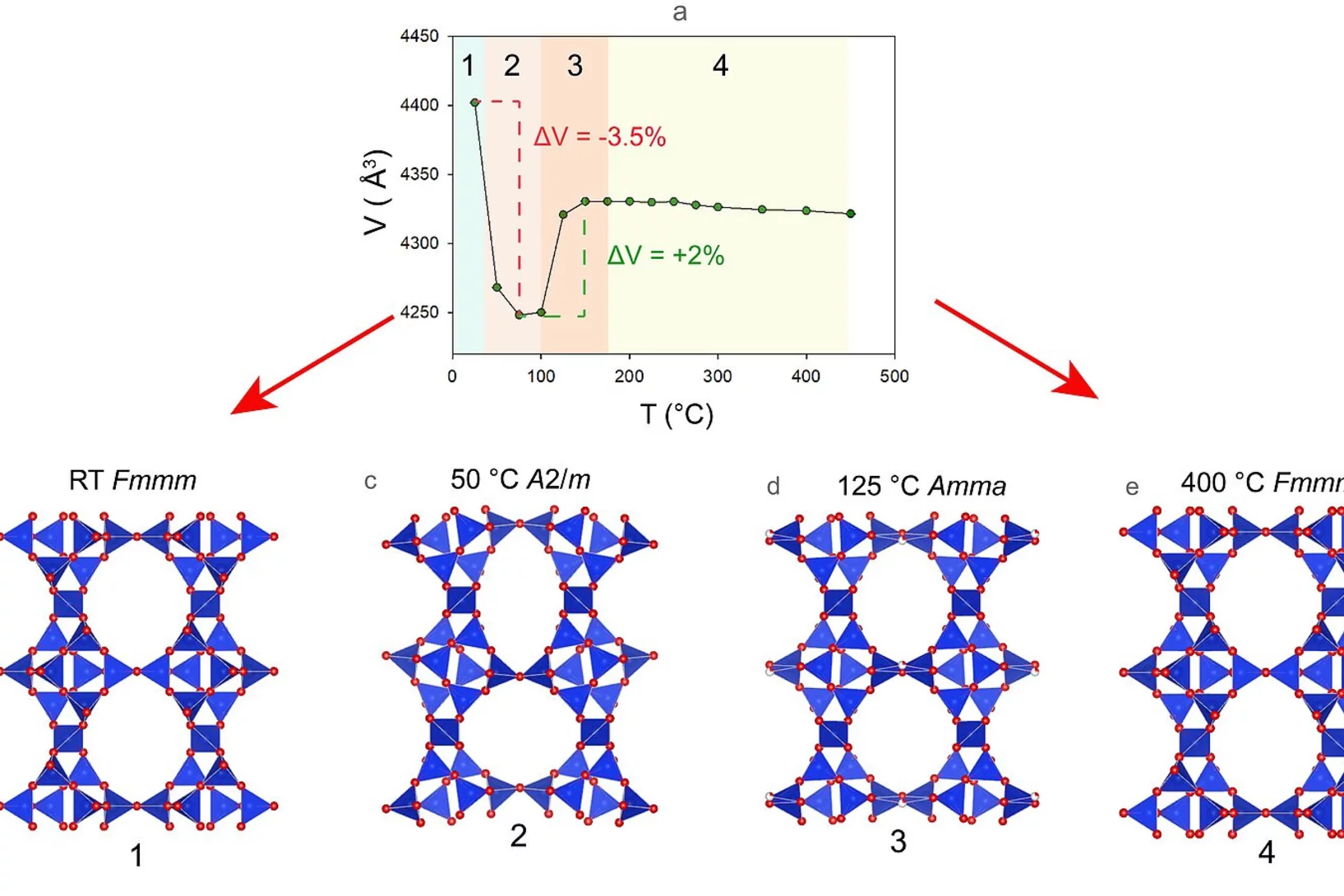
Zeolites are a class of shapely, colourful minerals with very special properties, making them omnipresent in our surroundings. They accelerate chemical reactions, absorb hazardous contaminants and water to a high degree, for example. Their only limitation is that they usually lose their peculiar crystalline structure at high temperatures. Now researchers at the University of Bern have found an unexpected exception.
Yanting Qian has received the MSc/PhD competition award in the FISA2022-EURADWASTE'22 conference
Yanting Qian has received the MSc/PhD competition award in the FISA2022-EURADWASTE'22 conference. She works on the retention of redox-sensitive Tc on Fe-bearing clay minerals.
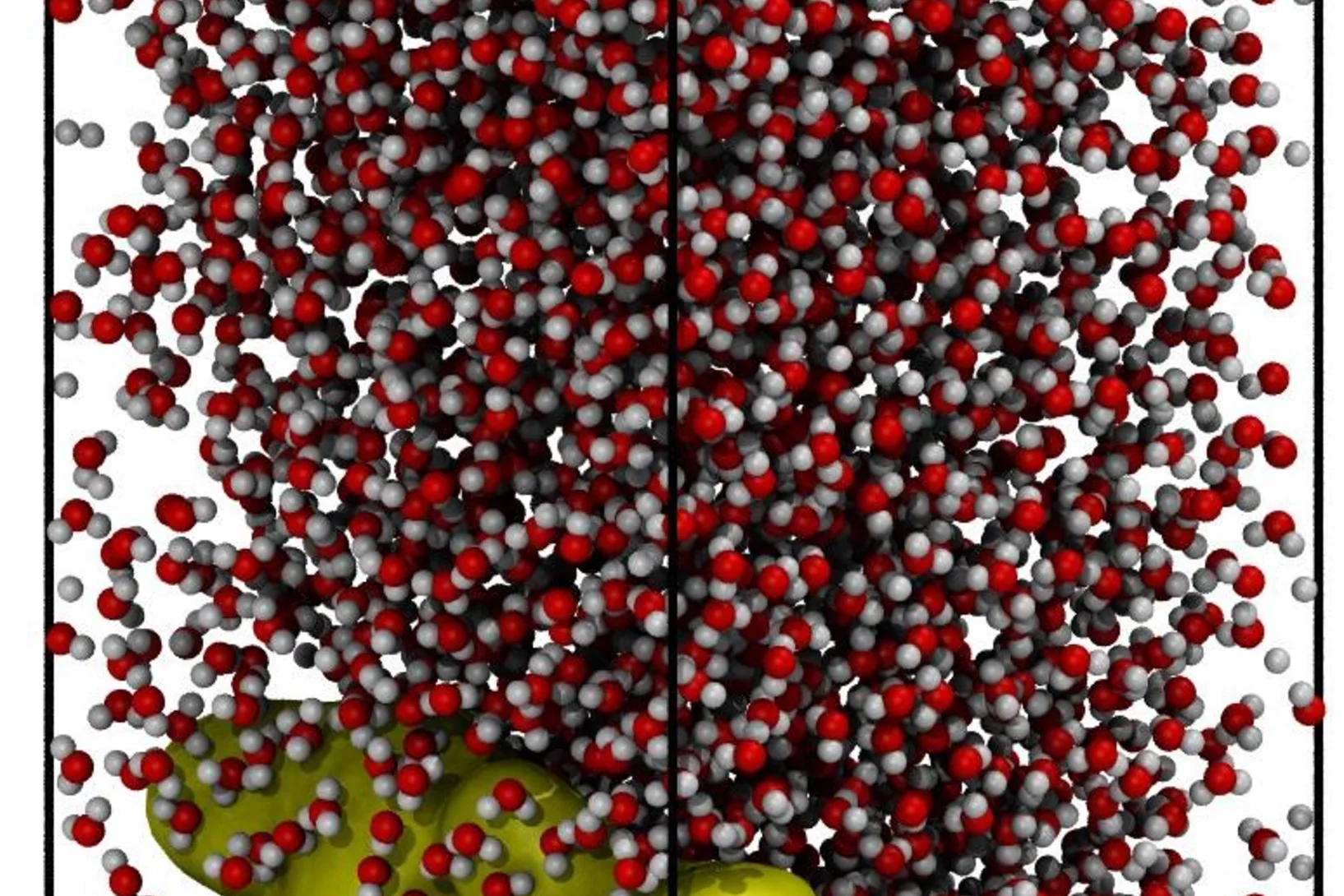
Deciphering the molecular mechanism of water boiling at heterogeneous interfaces
Water boiling control evolution of natural geothermal systems is widely exploited in industrial processes due to the unique non-linear thermophysical behavior. Even though the properties of water both in the liquid and gas state have been extensively studied experimentally and by numerical simulations, there is still a fundamental knowledge gap in understanding the mechanism of the heterogeneous nucleate boiling controlling evaporation and condensation. In this study, the molecular mechanism of bubble nucleation at the hydrophilic and hydrophobic solid–water interface was determined by performing unbiased molecular dynamics simulations using the transition path sampling scheme. Analyzing the liquid to vapor transition path, the initiation of small void cavities (vapor bubbles nuclei) and their subsequent merging mechanism, leading to successively growing vacuum domains (vapor phase), has been elucidated. The simulations reveal the impact of the surface functionality on the adsorbed thin water molecules film structuring and the location of high probability nucleation sites.