Present day technology relies to a large extend on lithium ion batteries for short-term storage of electrical power. This means that even minor improvements on this field have a large absolute impact. Our contribution here is the investigation of the Li distribution in the working electrode and on its surface during operation.
The method of choice is neutron reflectometry, which can deliver depth-profiles of the chemical composition of layered system with nm resolution. Especially that fact that this is possible even in systems obscured by containers and contacts allows for a continuous and non-destructive investigation of the composition as a function of time or externally applied voltage or currant. Last but not least, the time scale of NR of about 1 min is sufficiently smaller than the charging and discharging times of batteries.
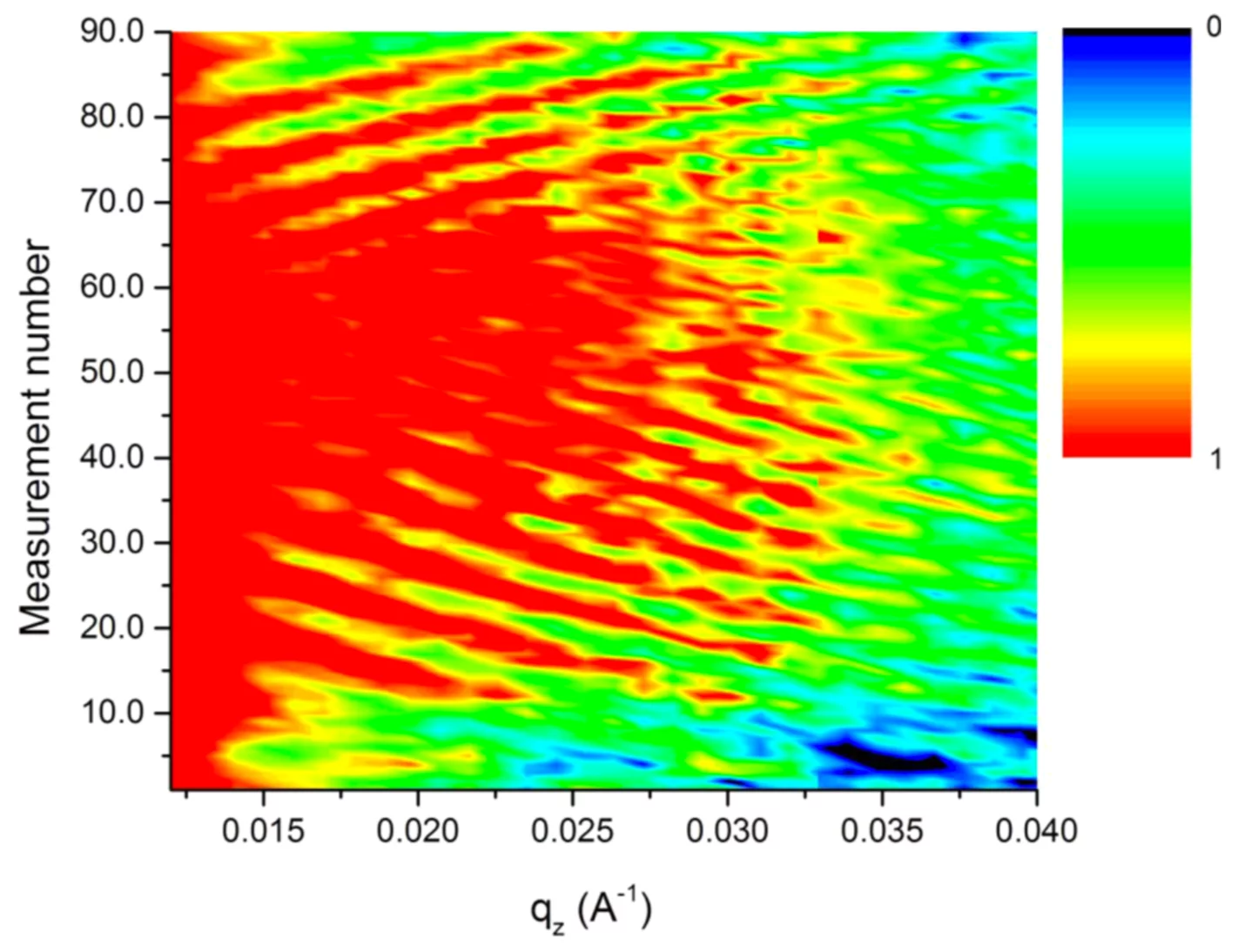
For the moment we concentrate on lithium silicon batteries because of their high Li and energy storage capacity. The disadvantage of this system is the huge volume change (up to 400%) upon charging. We managed to follow lithiation and de-lithiation of various samples cycled up to 7 times with a variety of currents and charging regimes. Figure 1 shows the change in neutron reflectivity as a function of Li content for one cycle. The prominent fringes are caused mainly from the total thickness of the electrode, their compression or expansion is thus a direct measure of its expansion. The exact Li distribution within the electrode can be extracted by fitting a model to the data.
We found that after some irreversible conditioning, for slow charging processes the volume expansion is reversible and almost linearly related to the amount of Li incorporated. But for fast charging, the initial expansion is much higher, than it slows down until it matches the value obtained with a slow process and the respective amount of Li.
Publications
- B. Jerliu, E. Huger, M. Horisberger, J. Stahn, H. Schmidt: "Irreversible lithium storage during lithiation of amorphous silicon thin film electrodes studied by in-situ neutron reflectometry", Journal of Power Sources 359 415-421 (2017), 10.1016/j.jpowsour.2017.05.095
- E. Huger, J. Stahn, P. Heitjans, H. Schmidt: "Neutron reflectometry to measure in situ the rate determining step of lithium ion transport through thin silicon layers and interfaces", Physical Chemistry Chemical Physics 21 16445-16450 (2019), 10.1039/c9cp01222b
- D. Uxa, B. Jerliu, E. Huger, L. Dorrer, M. Horisberger, J. Stahn, H. Schmidt: "On the Lithiation Mechanism of Amorphous Silicon Electrodes in Li-Ion Batteries", Journal of Physical Chemistry C 123 22027-22039 (2019), 10.1021/acs.jpcc.9b06011
- H. Schmidt, B. Jerliu, E. Huger, J. Stahn: "Volume expansion of amorphous silicon electrodes during potentiostatic lithiation of Li-ion batteries", Electrochemistry Communications 115 106738 (2020), 10.1016/j.elecom.2020.106738
- F. Strauss, E. Huger, P. Heitjans, T. Geue, J. Stahn, H. Schmidt: "Lithium Permeation through Thin Lithium-Silicon Films for Battery Applications Investigated by Neutron Reflectometry" Energy Technology 4 1582-1587 (2016), 10.1002/ente.201600209
- E. Huger, J. Stahn, H. Schmidt: "Neutron Reflectometry to Measure In Situ Li Permeation through Ultrathin Silicon Layers and Interfaces" Journal of the Electrochemical Society 162 A7104-A7109 (2015), 10.1149/2.0131513jes
Collaboration
- H. Schmidt, E. Hüger and B. Jerliu, TU Clausthal, Germany