Research Division Biology and Chemistry (BIO), surface chemistry Group, Head Markus Ammann.
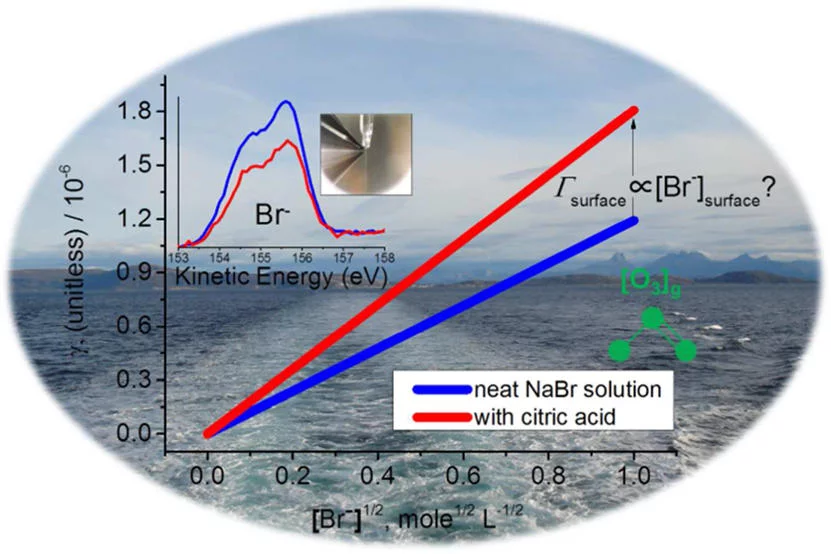
A more detailed understanding of the heterogeneous chemistry of halogenated species in the marine boundary layer is required. Here, we studied the reaction of ozone (O3) with NaBr solutions in presence and absence of citric acid (C6H8O7) under ambient conditions. Citric acid is used as a proxy for oxidized organic material present at the ocean surface or in sea spray aerosol. On neat NaBr solutions, the observed kinetics is consistent with bulk reaction limited uptake, and a second order rate constant for the reaction of O3 + Br- is 57±10 M-1 s-1. On mixed NaBr citric acid aqueous solutions the uptake kinetics was faster than that predicted by bulk reaction limited uptake and also faster than expected based on an acid catalyzed mechanism. X-ray photoelectron spectroscopy (XPS) on a liquid microjet of the same solutions at 1.0 x 10^3 - 1.0 x 10^4 mbar was used to obtain quantitative insight into the interfacial composition relative to that of the bulk solutions. It revealed that bromide anion becomes depleted by 30±10 % while the sodium cation gets enhanced by 40±20 % at the aqueous solution-air interface of a 0.12 M NaBr solution mixed with 2.5 M citric acid in the bulk, attributed to the role of citric acid as a weak surfactant. Therefore, the enhanced reactivity of bromide solutions observed in presence of citric acid is not necessarily attributable to a surface reaction but could also result from an increased solubility of ozone at higher citric acid concentrations. Whether the acid catalyzed chemistry may have a larger effect on the surface than in the bulk to offset the effect of bromide depletion also remains open.
Publication: http://dx.doi.org/10.1021/jp510707s
Further publications: LUC Homepage