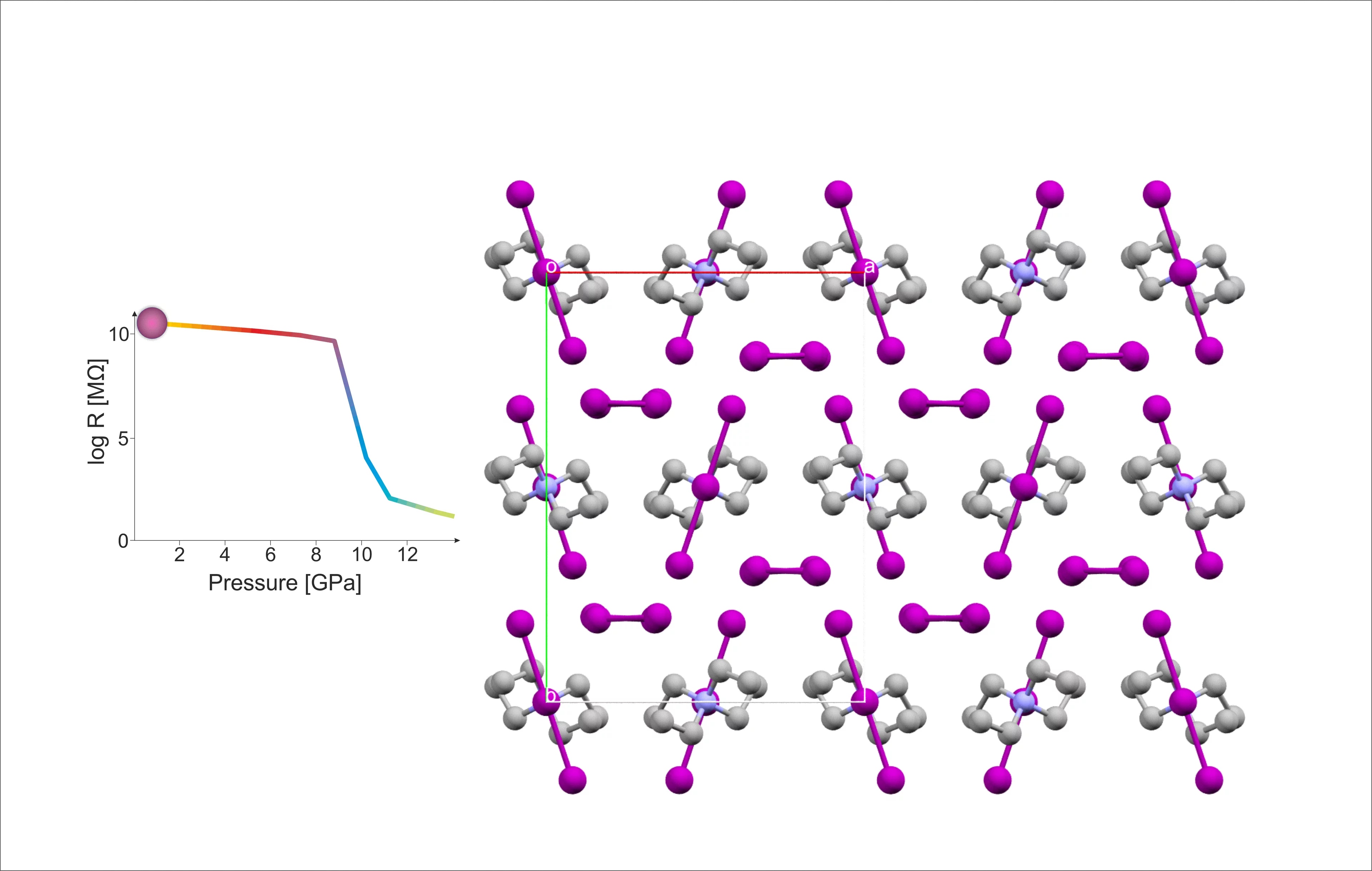
Organic polyiodides exhibit a very rich structural chemistry, comprising iodine chains with various lengths and geometries. In-situ X-ray diffraction experiments revealed that I2 and I3- in tetraethylammonium tridiodide (TEAI) undergo progressive addition reaction under high-pressure. They approach each other as the pressure is being increased and eventually form a polymeric chain at circa 10 GPa. Such condensed form was found to exhibit unusually high electrical conductivity for organic salts, whereas the ambient pressure structure is an insulator. First principle calculations show that the I-I bond in TEAI at ambient conditions is part ionic and part covalent in nature. High-pressure leads to an increase of the covalent character of I-I bond. Condensed iodine CT chains can conduct electricity through electron hopping only if the distance between two iodine units in polyiodide is lower than 3.4 Å. This structure-property correlation helps to validate the electronic properties of up-to-date synthesized polyiodides, as well as the design of the future iodine-rich functional materials.
Contact
Dr. Nicola Casati
Group Leader
PSI, Laboratory for Synchrotron Radiation - Condensed Matter
Forschungsstrasse 111, 5232 Villigen PSI, Switzerland
Telephone: +41 56 310 21 11
E-mail: nicola.casati@psi.ch
Original Publication
Pressure-induced Polymerization and Electrical Conductivity of Polyiodide
Poręba T., et al.
Angewandte Chemie intl. ed. (2019)
DOI: https://doi.org/10.1002/anie.201901178