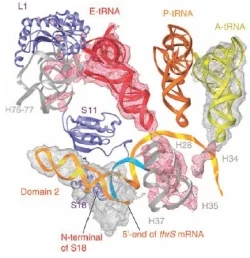
Ribosomes can be thought of as factories that build proteins from a set of genetic instructions. Translation relies on two selection processes: a) charging of tRNA by selection of the correct aminoacid to be covalently bound to it, b) the selection of the tRNA as specified by the codon of the mRNA. Aminoacyl-tRNA synthetases catalyse the first of these steps using hydrolysis of ATP. In the present study the ribosome of Thermus thermophilus was cocrystallised with initiator tRNAfMet and a structured mRNA fragment which codes for threonyl-tRNA synthetase. The thrS mRNA fragment consists of the translation operator domain flanked by two single stranded regions which constitute the ribosome binding site. Crystals containing functional ribosome in complex with initiator tRNAfMet and either thrS mRNA, mk27 mRNA from the bacteriophage T4, or in the absence of mRNA were obtained under similar experimental conditions. Highly complete and redundant data were collected from 300 to 5.5 Å at beamline X06SA. The electron densities derived from the diffraction images of the crystallised ribosome complexes suggest a general way in which mRNA control elements must be placed on the ribosome to perform their regulatory task.
Read full article
Read full article