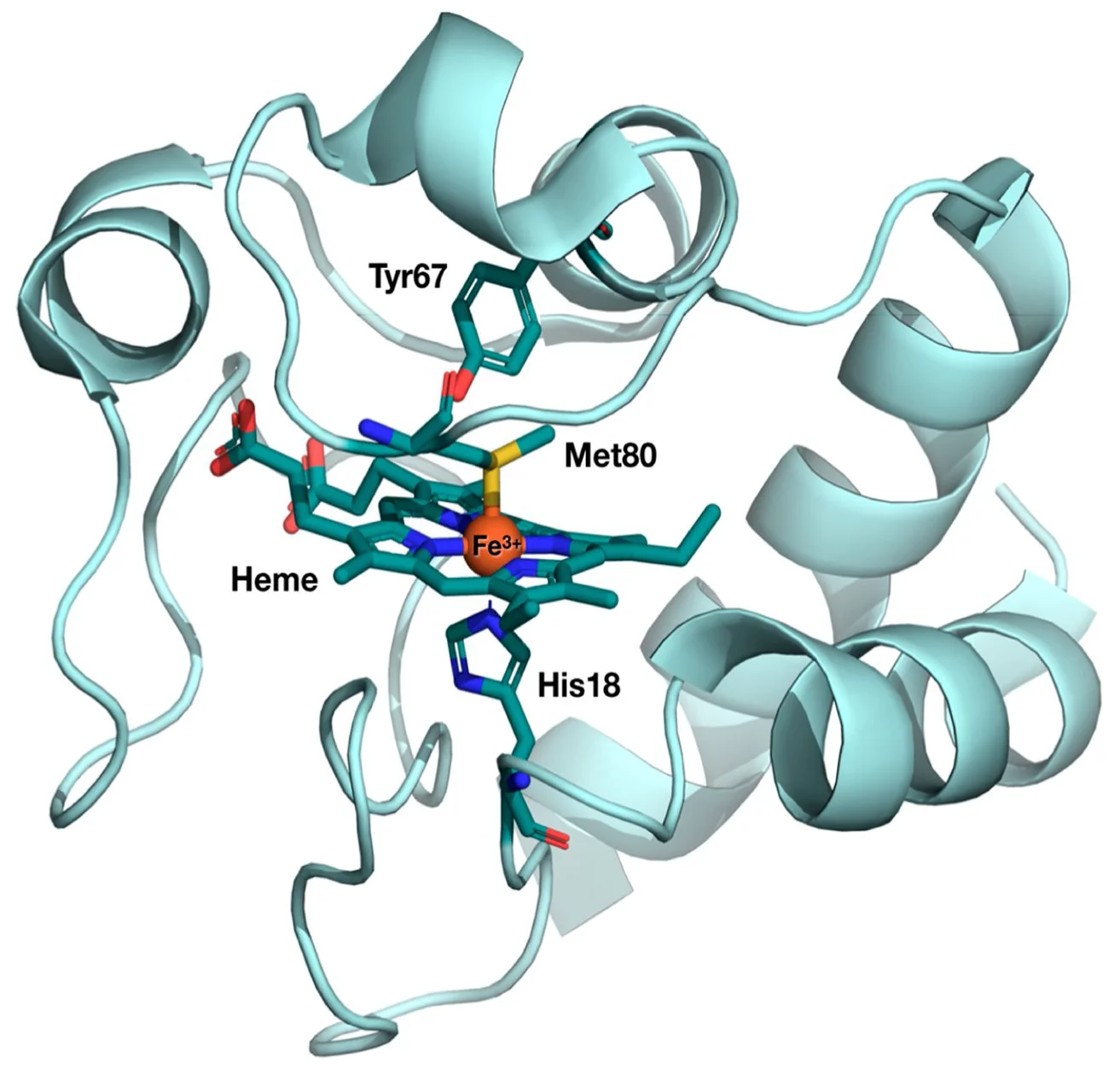
The first user experiment at SwissFEL took place in January 2019, when a team of researchers from Ecole Polytechnique Fédérale de Lausanne (EPFL) performed an experiment at the Alvra experimental station to probe the photo-induced electron transfer properties in cytochrome c. The goal was to understand how cytochrome c behaves, both structurally and electronically, as it performs its electron transfer function and the group used both X-ray absorption and emission spectroscopy to probe the Fe atom at the active centre of the protein. The structure of the protein, with the active centre highlighted, is shown below.
The results from this experiment were recently published in the Proceedings of the National Academy of Sciences (DOI: 10.1073/pnas.2009490117) and the results get to the heart of the structure-function relationship in biology: Specific structural changes in proteins are usually associated with specific functions. This is particularly the case with heme proteins, which perform a wide range of different biological functions, such as oxygen fixation and transport, and neurotransmission.
Specifically, cytochrome c is protein that is involved in the electron transport chain in the mitochondria, which is the pathway for the synthesis of ATP. Like all heme proteins, the active center of cytochrome c is the so-called “heme porphyrin”. The electron transfer properties of cytochrome c have been associated to a particular deformation of its heme center, so-called “ruffling”. In contrast, the “domed” deformation of the heme is the hallmark of respiratory proteins such as hemoglobin and myoglobin.
In a new experiment performed at the Alvra endstation at SwissFEL, a team of scientists led by Prof. Majed Chergui at EPFL’s School of Basic Sciences, with colleagues at the Paul Scherrer Institute and the European X-ray Free Electron Laser have found that cytochrome c also undergoes doming.
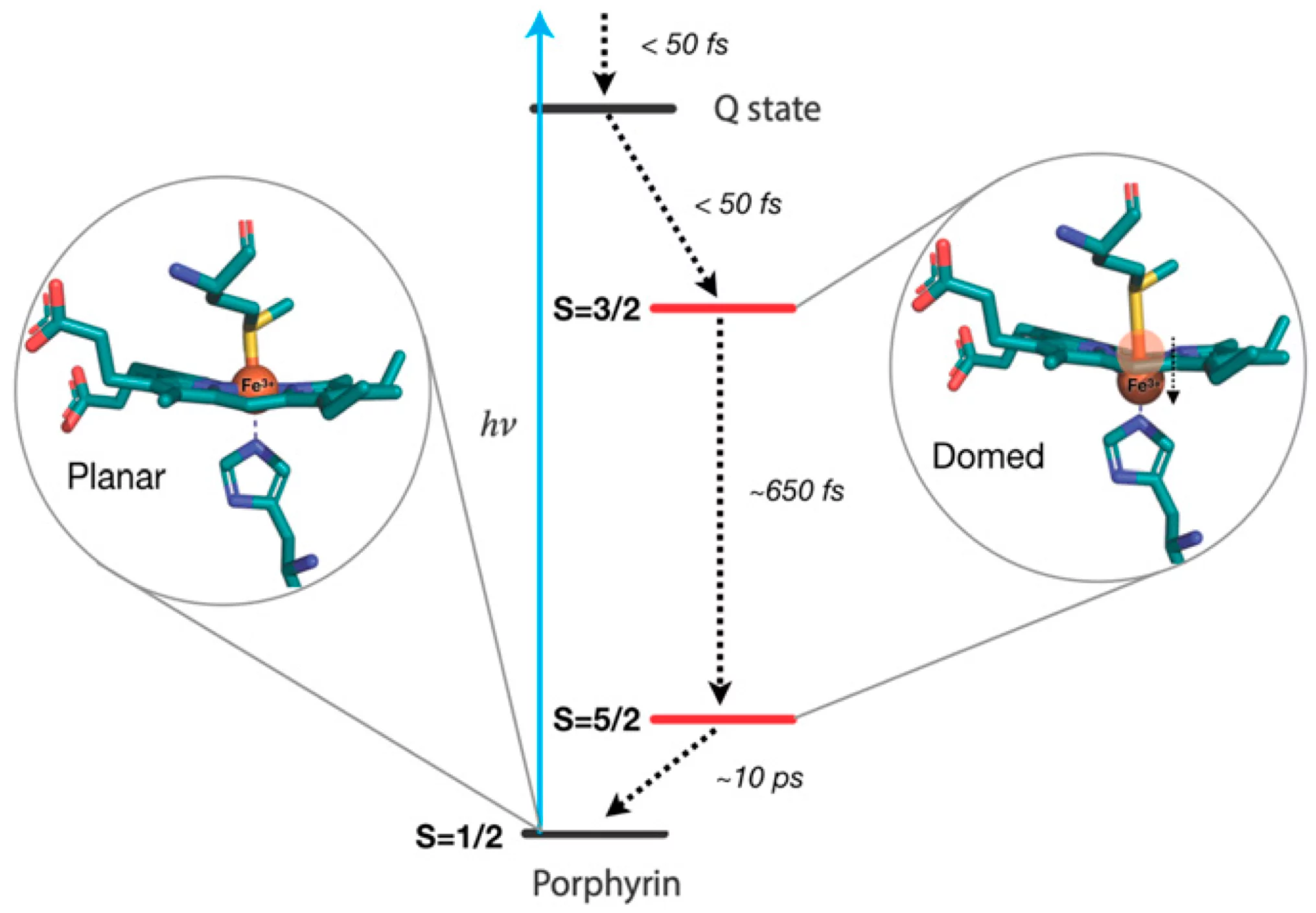
To carry out their study, the researchers used cutting-edge ultrafast X-ray spectroscopic techniques. They photoactivated the heme using ultrashort laser pulses, and monitored its evolution using another ultrashort X-ray pulse from SwissFEL to record X-ray absorption and X-ray emission of the protein as a function of time.
X-ray absorption at the Fe K-edge is sensitive to the structure of heme, while X-ray emission offers a fingerprint of its electronic states. By combining the two, the scientists have unambiguously determined that the system undergoes doming and goes back to its initial state via a cascade among Fe-centered spin states. The figure below shows the excited state energy relaxation processes in the protein, with the structure of the various spin-states emphasized.
“The conclusions of our work show that doming is a universal feature of all heme proteins and is not limited to respiratory ones (hemoglobin and myoglobin),” says Majed Chergui. “The question that arises now is the extent to which doming intervenes in the electron transfer function of cytochrome c.”
For further information on this result please contact Dr. Camila Bacellar (+41 56 310 4231) who is the lead author of the paper and a new SwissFEL instrument scientist at the Alvra experimental station.