Decarbonization of the energy system across different sectors using power-to-X concepts relies heavily on the availability of low-cost hydrogen produced from renewable power by water electrolysis. Polymer electrolyte water electrolysis (PEWE) is a promising technology for hydrogen (and oxygen) production for distributed as a well as centralized operation. The total cost of hydrogen is dominated by the electricity cost. Therefore, increase of conversion efficiency is pivotal in improving the commercial viability of electrolytically produced hydrogen. In this study, we investigate the prospects of improving conversion efficiency by reducing the membrane thickness from 200 to 50 micron and increasing the cell temperature from 60 to 120°C.
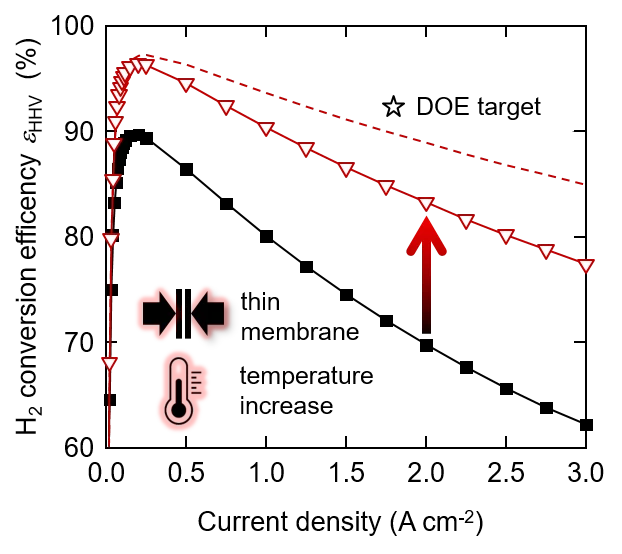
State of the art polymer electrolyte water electrolyzers typically use a perfluorinated proton exchange membrane of the Nafion type with a thickness of 150 to 200 micron and are operated at a cell temperature of up to 60°C. This conservative choice of materials and operating conditions ensures that the lifetime is in the range of ten thousands of hours. With a view to increasing the conversion efficiency, which corresponds to a decrease of the operating cell voltage, we investigated the effect of using a thinner membrane and increasing the cell temperature up to 120°C. We chose a Nafion membrane of 50 micron thickness. This is expected to reduce the ohmic resistance of the cell. The temperature increase is expected to lead to a reduction of the activation overpotential, in particular for the oxygen evolution reaction (OER), and an increase of the membrane conductivity. The figure shows the conversion efficiency of the water splitting reaction as a function of current density. The poor efficiency at low current density is a result of low faradaic efficiency due to gas crossover through the membrane. The curves go through a maximum and then decrease, which is a consequence of increasing overpotentials. As shown, with a combination of a 50 mm membrane and 120°C cell temperature, the efficiency can be increased by 15% at a current density of 3 A/cm2. Further reduction of the membrane thickness is limited by the gas diffusion through the membrane, which poses the risk of the formation of an explosive gas mixture (the lower explosion limit of H2 in O2 is 4%). The main overpotentials at 120°C with the thin membrane are related to the OER (0.34 V) and the ohmic resistance (0.36 V). The ohmic resistance is caused by the membrane, the interface between the anode catalyst layer and the titanium based porous transport layer, and electronic / contact resistances in the cell. If we could eliminate the latter, for example by a suitable coating on the titanium parts of the cell, the efficiency could be further enhanced (dashed line in the figure). The star indicates the development target by the US Department of Energy (DOE). We see that further development is necessary on the cell components to reach this goal. Moreover, the durability of the cell at elevated temperature needs to be studied in detail. Degradation phenomena are expected to be accelerated, which needs to be quantified to allow estimates of lifetime. This is the subject of ongoing studies.
Contact
PD Dr. Lorenz Gubler
Head Membranes & Electrochemical Cells Group
Paul Scherrer Institut
5232 Villligen PSI
Telephone: +41 56 310 26 73
E-mail: lorenz.gubler@psi.ch
Original Publication
Insight into Elevated Temperature and Thin Membrane Application for High Efficiency in Polymer Electrolyte Water Electrolysis
Steffen Garbe, Jonas Futter, Thomas J. Schmidt, Lorenz Gubler
Electrochimica Acta 377, 138046 (2021)
DOI: 10.1016/j.electacta.2021.138046
Acknowledgement
Swiss Federal Office of Energy (grant number SI/501603).