A system of evaporative cooling for Polymer Electrolyte Fuel Cells (PEFCs) has been developed at PSI, based entirely on one simple, yet effective change of one of the fuel cell components. Our team at the Electrochemistry Laboratory has demonstrated how this single change allows to operate a cell without the need of bulky and costly external humidifiers. Additionally, the proposed design has the potential to increase the power density of a PEFC stack by up to 35% due to the sparing of the space usually dedicated to the convective coolant circulation.
Polymer Electrolyte Fuel Cells (PEFCs) are considered an alternative to the carbon-based combustion engines, as they generate electricity out of hydrogen and oxygen, and release nothing but water. In a fuel cell, the reactant gases are brought in through gas channels on two opposite sides, one for the hydrogen (anode) and one for the air (cathode). Chemical reactions take place on the so-called catalyst layers (CL) to break up the reactant molecules on one hand and create water and electricity on the other. The two catalyst layers are very thin layers separated by a polymer membrane, which blocks electrons and only allows the passage of protons. This difference in the negative and positive charges pathways is what creates electricity. Membranes need to be well humidified to conduct protons and this is usually achieved by humidifying the reactant gases upstream from the cell using costly external humidifiers.
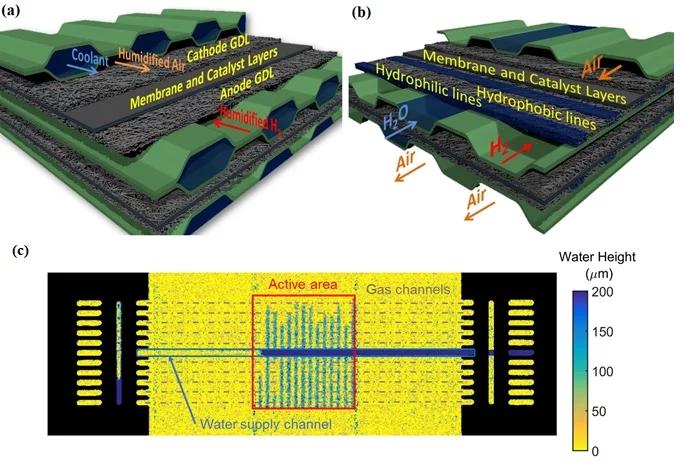
To reach the CL, the reactant gases in the channels have to diffuse through very thin porous media made of intertwined carbon fibers, and called gas diffusion layer (GDL). The GDL also helps transport the water produced by the chemical reaction from the CL to the gas channels, where it can be removed by the gas stream. To avoid retaining water droplets in the GDL’s small pores, which would block the pathways of the gases to the CL, the GDL is coated with a highly hydrophobic solution (very often, PTFE).
Last, the electrochemical reactions are on balance exothermic, and the heat produced must be removed to avoid overheating the cell. Usually this is achieved by flowing a coolant in internal circuits placed between two adjacent cells, at the expense of the cell’s compactness.
PEFC still face many challenges to reduce their size and cost before they can reach commercialization on a large scale. One of these challenges is to humidify the reactant gases without expensive external humidifiers. Another one is to remove the waste heat of the reactions without dedicating a consequent part of the PEFC volume to cooling only.
At PSI, a process has been developed to add a hydrophilic polymer on selected surfaces of the hydrophobic coating and transform the GDL into a succession of hydrophobic and hydrophilic zones. These zones of opposite wettability allow the separate transport of gases and water. We can use these advanced GDLs to build a fuel cell with evaporative cooling, where humidification and cooling are managed simultaneously, in a way that entirely removes the need for internal cooling circuit and for external humidifiers.
The modified GDL is placed on the anode side, with the hydrophilic/hydrophobic lines perpendicular to the gas channels. A small number of these channels are transformed into liquid water supply channels. The water brought in these channels is wicked directly by the hydrophilic lines, and spreads above several gas channels. Once in contact with the hydrogen flow, the water in the hydrophilic line evaporates, which provides the humidification and the cooling effect needed. Thanks to the constant supply of water, the hydrophilic lines are always filled, and high evaporation rates can be sustained as long as needed for different operating conditions. Meanwhile, the (very) hydrophobic lines remain dry pathways for the reactant gases and ensure a good operation of the cell.
We have demonstrated, using a 4 cm2 laboratory cell equipped with thermal management instrumentation, that our concept can indeed provide the levels of humidification and cooling power needed for the good operation of a fuel cell at 80°C, and potentially at higher temperatures. The concept is robust, provided the pressure of the water is well controlled, and the performance of the cell with evaporative cooling is similar to that of the cell without evaporative cooling, albeit with a small loss of efficiency at very high current densities. Fuel cell stacks equipped with this concept could be up to 33% more compact, which would mean, after accounting for the small loss in efficiency, that an increase of the global power density of up to 35% of the cell would be possible. In addition the removal of the external humidifiers, and the simplification of the entire system would make a fuel cell equipped with this evaporative cooling concept an attractive option for an automotive powertrain.
Contact
Dr. Pierre Boillat
Head of Neutron Imaging of Electrochemical Systems Group
Paul Scherrer Institut
5232 Villigen PSI
Switzerland
Telephone: +41 56 310 27 43
E-mail: pierre.boillat@psi.ch
Original Publication
Enabling High Power Density Fuel Cells by Evaporative Cooling with Advanced Porous Media
M. Cochet, A. Forner-Cuenca, V. Manzi-Orezzoli, M. Siegwart, D. Scheuble, P. Boillat
J. Electrochem. Soc. 167, 084518 (2020)
DOI: 10.1149/1945-7111/ab8e82
Acknowledgement to Funding Agency
The Swiss Competence Center for Energy Research: Efficiency in Mobility (SCCER Mobility) and the Swiss National Science Foundation (project grants no. 143432 and 172474) are gratefully acknowledged for their financial support.