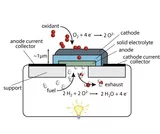
The harsh operational conditions of Solid Oxide Fuel Cells (T: typically 800-1000°C, highly oxydizing resp. reducing atmosphere) impose stringent requirements on the materials' selection. The electrochemically active heart of an SOFC consists of 3 main functional compartments: porous and electronically conductive electrodes (anode & cathode) separated by a dense, gas tight electronically insulating electrolyte. Oxygen conductive electrolytes include solid solutions of fluorite type ZrO2 and CeO2 as well as perovskites like doped lanthanum gallates. Possible enhancement of transport / thermodynamical properties by control of the microstructure on a nm-scale is explored. Novel perovskites, preferably mixed ionic and electronic conductors with high electrocatalytic activity are within the scope of research activity towards enhanced electrode materials for thin film technology based micro SOFCs as high potential future mobile energy sources.