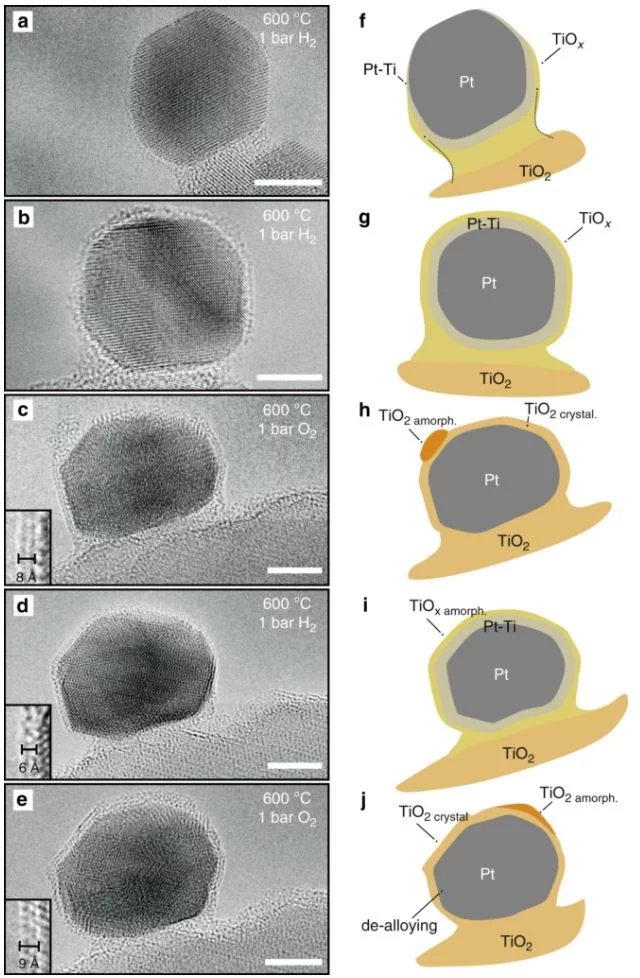
Heterogeneous catalysts play a pivotal role in the chemical industry. The strong metal-support interaction (SMSI), which affects the catalytic activity, is a phenomenon researched for decades. However, detailed mechanistic understanding on real catalytic systems is lacking. Here, this surface phenomenon was studied on an actual platinum-titania catalyst by state-of-the-art in situ electron microscopy, in situ X-ray photoemission spectroscopy and in situ X-ray diffraction, aided by density functional theory calculations, providing a novel real time view on how the phenomenon occurs. The migration of reduced titanium oxide, limited in thickness, and the formation of an alloy are competing mechanisms during high temperature reduction. Subsequent exposure to oxygen segregates the titanium from the alloy, and a thicker titania overlayer forms. This role of oxygen in the formation process and stabilization of the overlayer was not recognized before. It provides new application potential in catalysis and materials science.
The webpage can be accessed at Nature Communications and is linked prominently on the journal homepage (https://www.nature.com/ncomms/) and a dedicated Editors’ Highlights page (https://www.nature.com/ncomms/editorshighlights).