In the PSI hydrothermal gasification process for wet biomass salt separation turned out to be a crucial step. It is necessary to remove the inorganic salts from the feed stream to avoid catalyst poisoning and furthermore it is a chance to recover nutrients like Ca, Na, K, N, P and S which could be used as fertilizers in biomass production. As the separation efficiency of the process is determined by the phase behavior of the solutions, a detailed knowledge is needed. Our investigations in this field are divided in two major parts, namely thermodynamics and in situ-spectroscopy.
The thermodynamics of aqueous salt solutions are studied with isochoric High Pressure Differential Scanning Calorimetry (HP-DSC). To obtain corresponding pressure-temperature data we use a small but dedicated autoclave. Furthermore phase transitions are visually observed under isothermal or isobaric conditions using a high pressure cell equipped with sapphire windows.
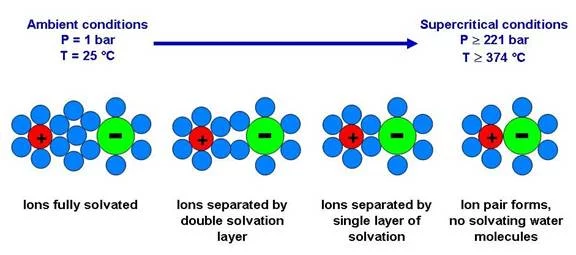
Since the macroscopic behavior of salt solutions in the proximity of the critical point of water is strongly determined by changes in the microscopic structure of the solution, we will use spectroscopic methods to gather knowledge in this field. Especially the formation of ion-pairs in the proximity of the critical point of water is of interest. For our in situ-spectroscopic investigations a special cell was constructed. This cell is equipped with thin diamond windows which allow measuring XAS-spectra at the S and P K-edge. Measurements will be conducted at the Phoenix beamline of the Swiss Light Source (SLS).
The pressure and temperature range of our investigations will be 200-400°C and 1-350 bars.
Project Contact
Biomass related aqueous salt solutions at high pressures and temperatures
Prof. Dr. Frédéric Vogel
+41 56 310 5735
frederic.vogel at psi.ch