In a joint project with the Center for Cellular Imaging and NanoAnalytics (C-CINA) at the University of Basel and the Lab. for biomolecular research at PSI we are developing tools and methods for the preparation of protein crystals for dynamic studies at XFEL sources. The project is financed by the Swiss Nanoscience Institute.
Serial femtosecond crystallography based on X-ray free-electron laser sources (XFELs) provides new opportunities for structural sciences, in particular for the time-resolved investigation of dynamic processes [1-3]. Even though after every ultrafast shot of a very intense coherent laser pulse the sample is destroyed due to Coulomb explosion, X-ray diffraction data can be collected in the so-called ‘diffract-before-destroy’ regime.
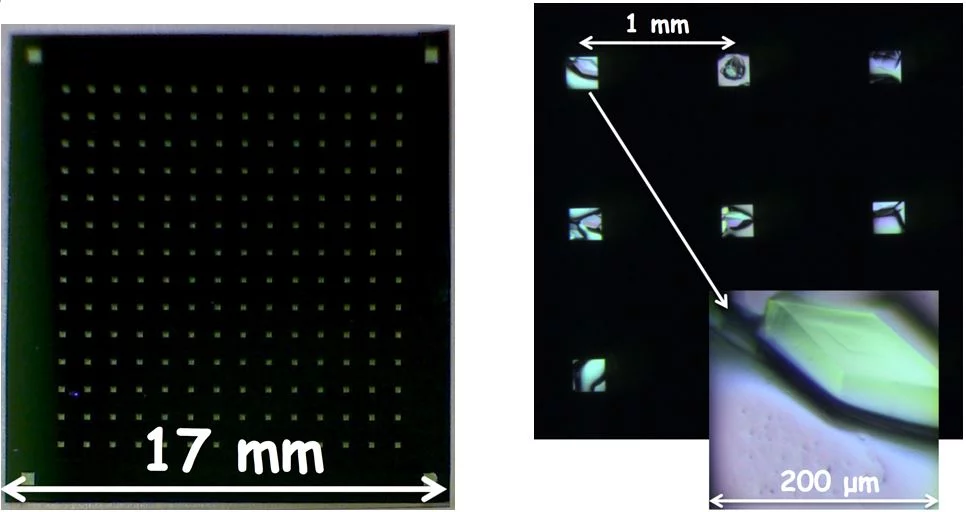
High efficiency measurements at XFELs require high density and very well ordered deposition of the biological sample – usually two dimensional (2D) or three dimensional (3D) crystals of proteins – on a substrate. The mount, which has an array of ultrathin windows (Fig. 1), is positioned on a moveable stage allowing it to be scanned by the (fixed) laser beam. This approach gives much higher hit rates and much lower sample consumption than the liquid jets commonly used to inject a large number of small crystals into the beam.
Microfabricated solid supports (fixed targets) filled with crystals protected from dehydration, allow measurements to be performed at room temperature, enabling structure determination of macromolecules in a close-to-native environment at high resolution [4].
Well-established microfabrication technology based on materials such as silicon and silicon nitride makes it possible to create ultrathin, low background packaging, that is especially useful for supporting 3D nanocrystals or 2D crystals, which have too low diffraction yields for reasonable measurements to be made using synchrotron radiation.
Further development towards highly X-ray transparent, less fragile, easy to mass-fabricate devices is required to allow future cost-efficient high throughput measurements at FELs. The main aim of this project is to improve sample preparation methods for XFEL-based protein nanocrystallography. In particular, we develop supports optimized for protein crystal preparation, to minimize sample consumption by adapting the liquid handling system and to perform proof-of-concept pump-probe experiments on solid supports [5,6].
References:
[1] I. Schlichting, IUCrJ, 2015, 2, 246-255
[2] M. Frank et al., IUCrJ, 2014, 1, 95–100
[3] S. Boutet et. al, Science 337, 2012, 362
[4] A. Zarrine-Afsar et al., Acta Cryst. 2012, D68, 321-323
[5] N. Opara et al., "Direct protein crystallization on ultrathin membranes for diffraction measurements at X-ray free electron lasers." J. Appl. Cryst. (2017) 50, 909-918.
[6] N. Opara et al., "Demonstration of femtosecond X-ray pump X-ray probe diffraction on protein crystals". Structural Dynamics 5, 054303 (2018).
High efficiency measurements at XFELs require high density and very well ordered deposition of the biological sample – usually two dimensional (2D) or three dimensional (3D) crystals of proteins – on a substrate. The mount, which has an array of ultrathin windows (Fig. 1), is positioned on a moveable stage allowing it to be scanned by the (fixed) laser beam. This approach gives much higher hit rates and much lower sample consumption than the liquid jets commonly used to inject a large number of small crystals into the beam.
Microfabricated solid supports (fixed targets) filled with crystals protected from dehydration, allow measurements to be performed at room temperature, enabling structure determination of macromolecules in a close-to-native environment at high resolution [4].
Well-established microfabrication technology based on materials such as silicon and silicon nitride makes it possible to create ultrathin, low background packaging, that is especially useful for supporting 3D nanocrystals or 2D crystals, which have too low diffraction yields for reasonable measurements to be made using synchrotron radiation.
Further development towards highly X-ray transparent, less fragile, easy to mass-fabricate devices is required to allow future cost-efficient high throughput measurements at FELs. The main aim of this project is to improve sample preparation methods for XFEL-based protein nanocrystallography. In particular, we develop supports optimized for protein crystal preparation, to minimize sample consumption by adapting the liquid handling system and to perform proof-of-concept pump-probe experiments on solid supports [5,6].
References:
[1] I. Schlichting, IUCrJ, 2015, 2, 246-255
[2] M. Frank et al., IUCrJ, 2014, 1, 95–100
[3] S. Boutet et. al, Science 337, 2012, 362
[4] A. Zarrine-Afsar et al., Acta Cryst. 2012, D68, 321-323
[5] N. Opara et al., "Direct protein crystallization on ultrathin membranes for diffraction measurements at X-ray free electron lasers." J. Appl. Cryst. (2017) 50, 909-918.
[6] N. Opara et al., "Demonstration of femtosecond X-ray pump X-ray probe diffraction on protein crystals". Structural Dynamics 5, 054303 (2018).