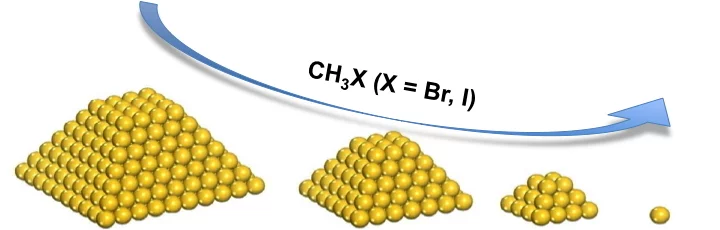
Gold particles supported on carbon when subjected to a flow of methyl iodide or bromide redisperse from large ensembles to single atoms and/or dimers of gold. Methyl halide oxidizes gold leading to gradual particle dissolution. The process could be carried out at temperatures as low as 50 °C. The excess of halide could be removed by a post-treatment of the material with 1%H2O/H2, which does not influence the metal dispersion. This remarkable transformation opens the possibility of re-activating gold catalysts that lost their performance due to metal particles sintering.
The finding was proposed based on a combination of in situ (XAS), ex situ characterization (aberration corrected HAADF-TEM, XRD and XPS) and kinetic measurements. The work was a combined effort from Queen’s University Belfast (UK), Paul Scherrer Institute (Switzerland), Lehigh University (USA) and Cardiff University (UK)
Publication:http://dx.doi.org/10.1002/anie.201102066
Further publications:LBK Publications