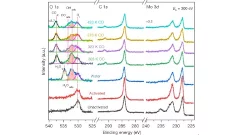
The water–gas shift reaction, leading to the production of hydrogen and carbon dioxide from carbon monoxide and water, is of paramount industrial importance. High activity and stability of the catalysts, typically employed in fuel cells, are important parameter to consider to properly design new materials.
In this work, combining reactivity tests with microscopy and spectroscopy, it has been demonstrated that the co-presence of platinum isolated atoms and nanoclusters supported on molybdenum carbide allows the protection of the support, leading to high stability and high metal-normalized turnover number of 4,300,000 moles of hydrogen per mole of platinum. XPS measurements carried out at the In Situ Spectroscopy beamline (X07DB) have helped to follow the reaction after activation of the catalyst, contributing to understand the reaction mechanism.
Contact:
Dr. Artiglia Luca Prof. Dr. Jeroen A. van Bokhoven
Senior Scientist Laboratory head
Paul Scherrer Institute PSI, 5232 Villigen PSI, Switzerland
Telephone: +41 56 310 46 68
E-mail: luca.artiglia@psi.ch
Title: A stable low-temperature H2-production catalyst by crowding Pt on α-MoC
Authors: Xiao Zhang, Mengtao Zhang, Yuchen Deng, Mingquan Xu, Luca Artiglia, Wen Wen, Rui Gao, Bingbing Chen, Siyu Yao, Xiaochen Zhang, Mi Peng, Jie Yan, Aowen Li, Zheng Jiang, Xingyu Gao, Sufeng Cao, Ce Yang, A. Jeremy Kropf, Jinan Shi, Jinglin Xie, Mingshu Bi, Jeroen A. van Bokhoven, Yong-Wang Li, Xiaodong Wen, Maria Flytzani-Stephanopoulos, Chuan Shi, Wu Zhou, Ding Ma
Journal: Nature, 589, 396–401, 2021