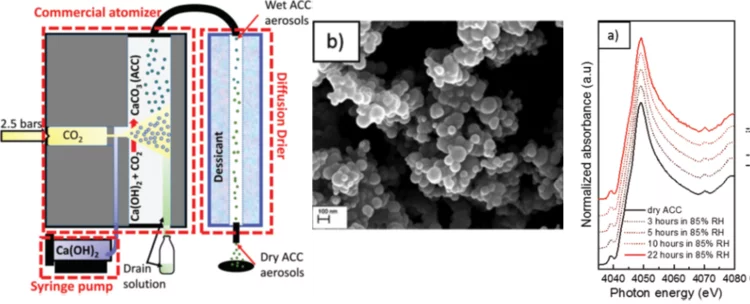
Aerosol-based synthesis of pure and stable amorphous calcium carbonate
Calcium carbonates are key materials to biomineralization, they are frequently used in industrial applications and also for carbon capture technologies. Finally, they serve as an important model system to test novel nucleation theories. Calcium carbonate crystalizes in a multi-step process, where amorphous calcium carbonate (ACC) is the most important precursor in the crystallization process. Existing synthesis protocols generate ACC of different stability and purity. To improve our mechanistic understanding of carbonate crystallization, reactivity and polymorph formation, the reproducible synthesis of clean and stable ACC is an important, and yet unresolved step. Here we use the fast reaction of CO2 with calcium hydroxide in airborne aerosols to reproducibly create pure and stable ACC, which may serve as a well-defined starting material for further chemical processing.
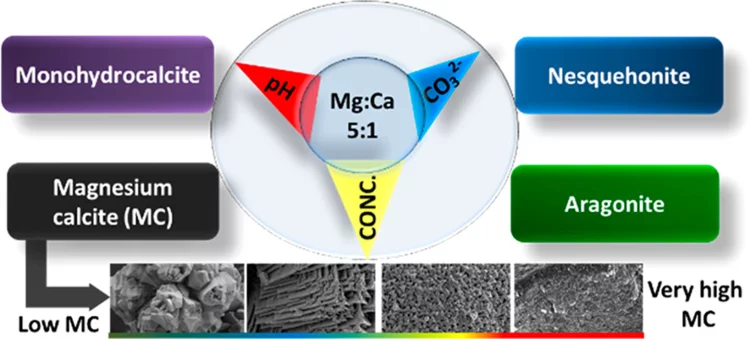
Tuning the magnesium content in magnesium rich-calcites
Magnesium rich calcites are important functional biominerals. For example, they can be found in protective shells or eye lenses. Natural organism provide a surprisingly high degree of control on the amount of magnesium incorporation into calcites by yet not well understood mechanisms. Understanding such control mechanism is important when designing bio inspired functional materials. Here we systematically explore the impact of thermodynamic parameters on the degree of magnesium incorporation into calcite. In particular, we identify the thermodynamic conditions, where very high magnesium rich calcites (50% Mg/50% Ca) forms under ambient conditions of temperature and pressure. This is an important finding for geochemistry: Very high magnesium rich calcite is believed to be the precursor for dolomite. Despite its frequent occurrence in nature, its unknown formation pathway remains one of the big mysteries in geochemistry.
Investigation of anionic redox activities in organic-based electrode for Li-ion batteries
To date the electrochemical activity of battery materials was always relying in the oxidation/reduction of cationic redox (change of oxidation state of transition metals generally). However, recently, it was established in new cathode materials (so call Li-rich cathode) that the oxygen from the crystal lattice might also play the role of anionic redox center leading to enhance then the specific charge of battery materials.
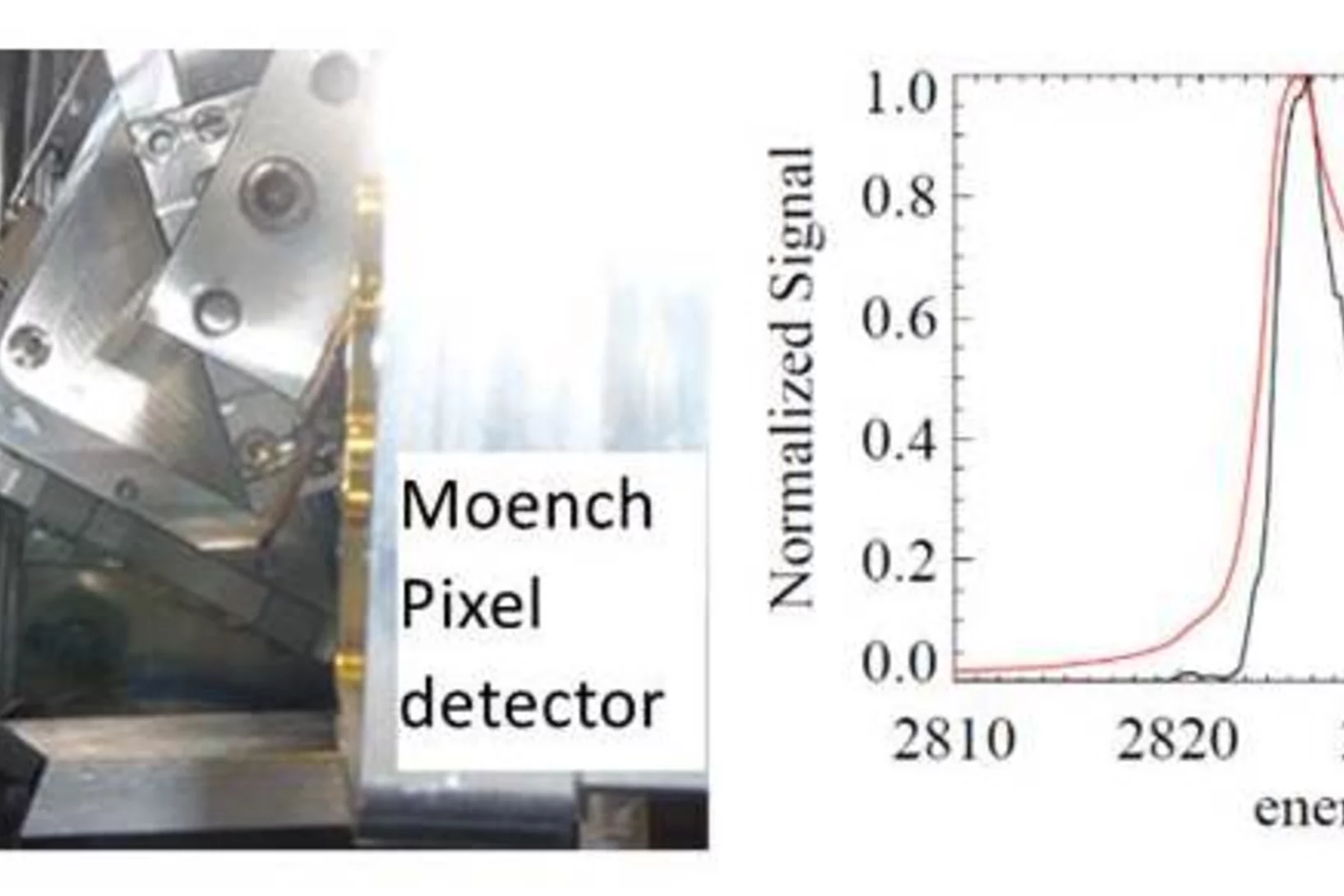
Von Hamos spectrometer for tender energies open to users
A new compact von Hamos spectrometer for tender x-rays (current energy range 2.25-4.5 keV), is now available for emission spectroscopy. This spectrometer allows analyzing the energetic composition of fluorescent light from the sample. It provides research opportunities for emission spectroscopy, and RIXS on the K (P-Sc), L (Zr-Cs) and M (Ir-Fr) absorption edges.
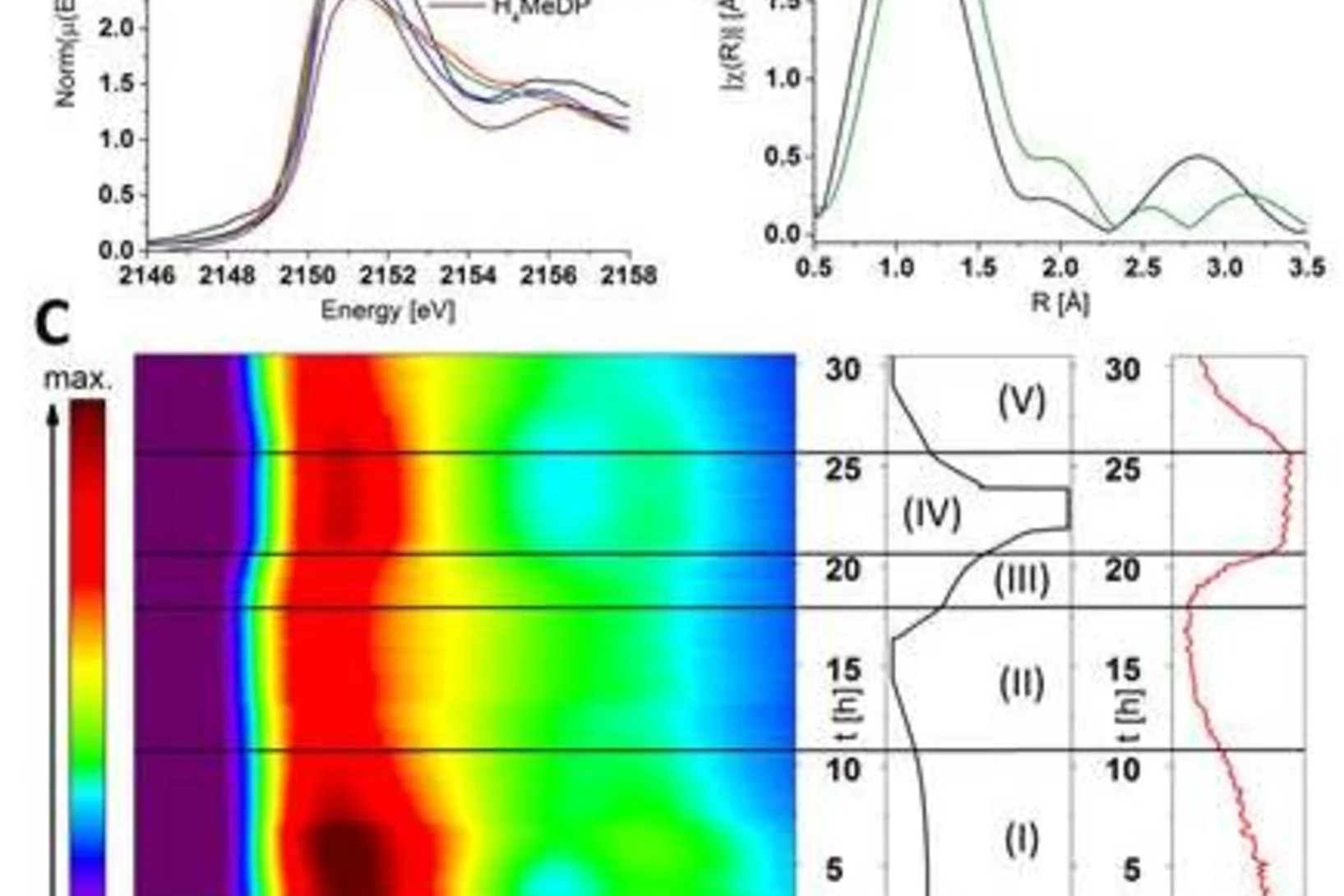
Amorphous CaCO3: Influence of the Formation Time on Its Degree of Hydration and Stability
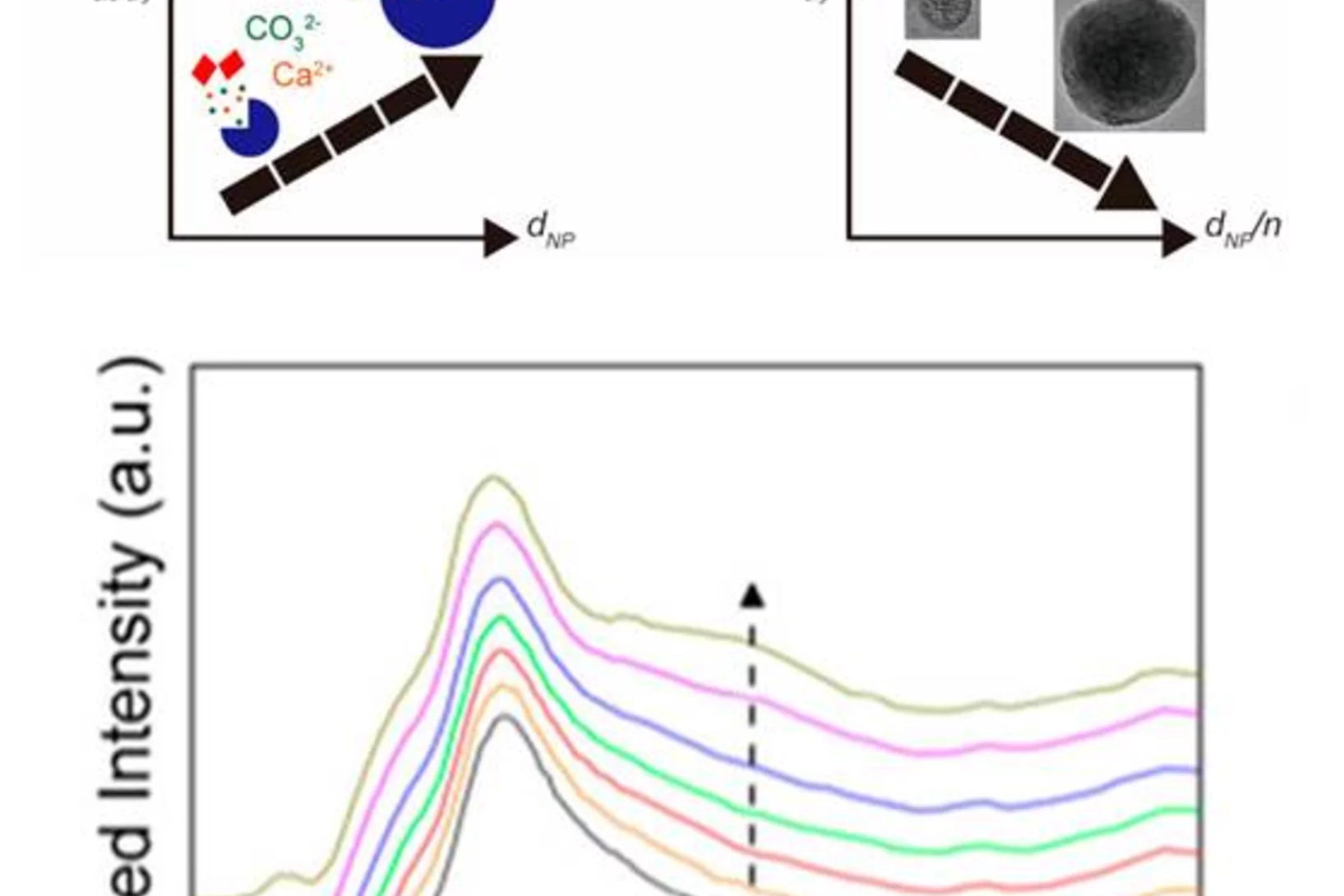
Carbonate minerals serve as reservoir for CO2 in the global CO2 cycle, as biomineral in animal skeletons and shells of marine animals, and are used in carbon capturing techniques. Moreover, they serve as an important model system in crystallization studies, and have important commercial applications, for example as fillers. Researchers from EPFL and PSI developed a new methodology to study the crystallization of CaCO3 that offers both high temporal and spatial resolution, which is the key challenge in elucidating early stages of crystallization. Using X-ray absorption spectroscopy and other techniques it could be demonstrated that the degree of hydration of amorphous CaCO3 increases during its growth. As a result of the increasing degree of hydration, the stability of the resulting amorphous particles against solid-state crystallization decreases.
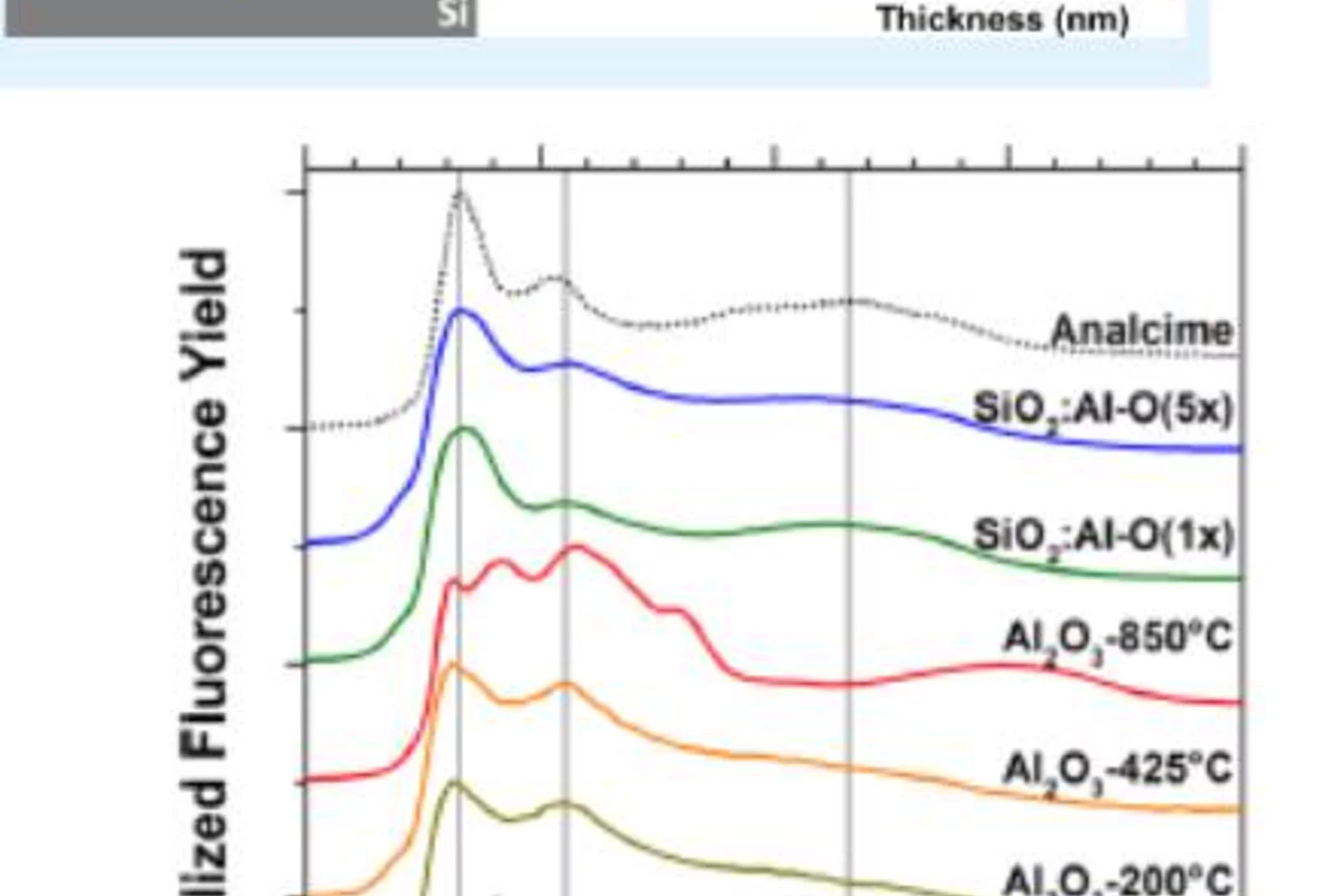
The negative charge density in Al-O monolayers on SiO2 surfaces
Single atomic layers of aluminum oxide embedded in SiO2 thin films, play an important role for the design of carrier-selective passivating contacts for high efficiency silicon based photovoltaic applications. Researchers from the Australian National University (ANU, Canberra, Australia), the Karlsruhe Institute of Technology (IT, Karlsruhe, Germany) and PSI have used synchrotron radiation to reveal the bonding configuration and local atomic surrounding of the Al-atoms in such surface oxide layers. The results corroborates theoretical calculations and contribute to a new model to explain the origin of the negative fixed charge in the Al-O/SiO2 stack, which has promising properties for a carrier-selective passivating contact for future silicon solar cells.
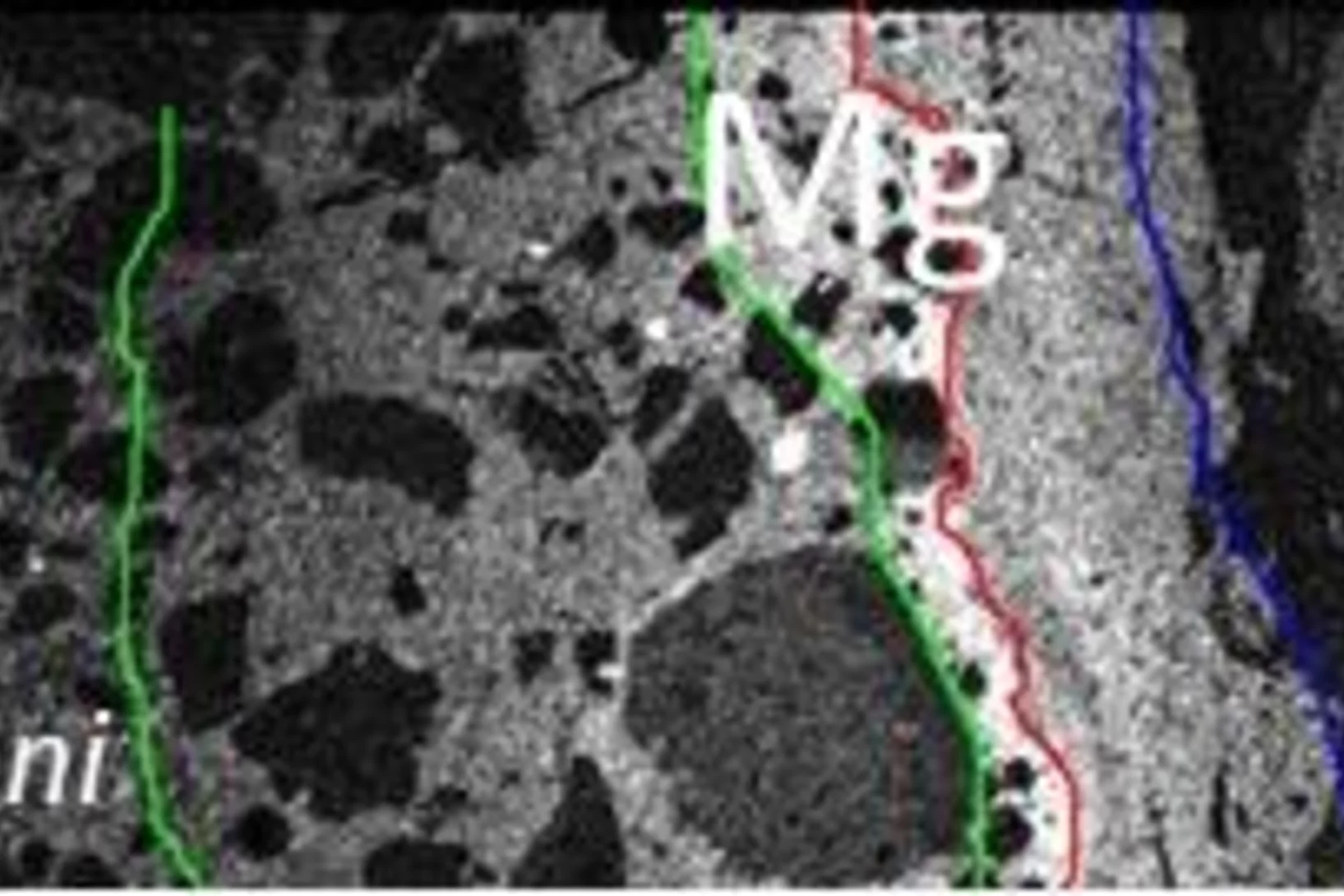
When man-made stones meet natural rocks – Shedding light on Mg-rich phases appearing at the interface between concrete and clay
Claystones and cement-based materials are key materials for safe disposal of radioactive waste in deep geological repositories. In Switzerland, Opalinus Clay, was selected as geological host material. At the Mont Terri rock laboratory the alteration of cement in contact with the natural clay is studied in a several years lasting experiment. The formation of different magnesium containing phases at the interface was studied using X-ray absorption micro-spectroscopy at the PHOENIX beamline of the Swiss Light Source (SLS).
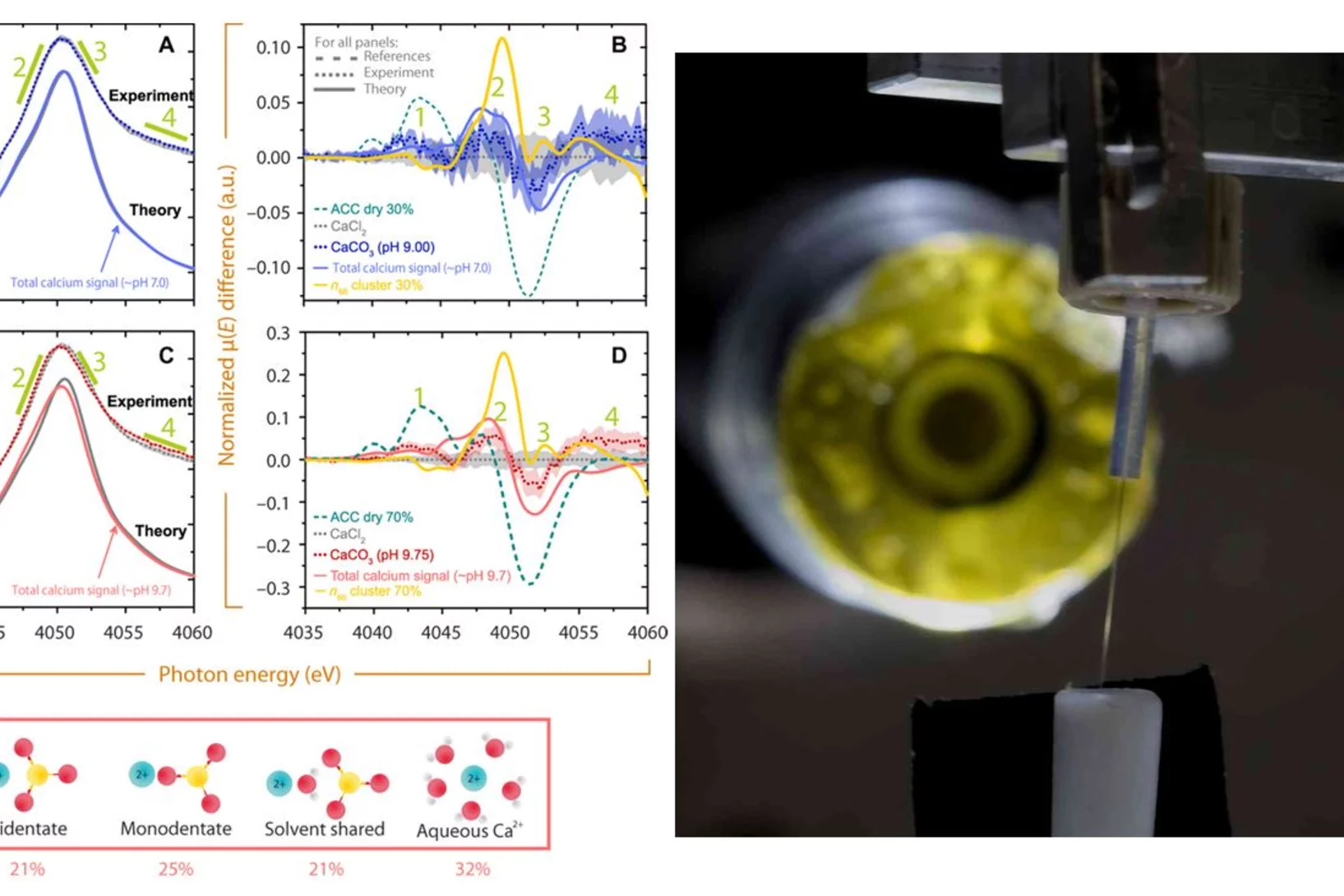
Are supersaturated calcium carbonate solutions classical or non-classical ?
Classical theory predicts that supersaturated carbonate solutions consist mostly of ions and ion pairs, with a small number of larger clusters present in the solution. The population of the different sized clusters in a solution is solely defined by the cluster’s size dependent Free Energy. If clusters are large enough they serve as nucleation germs for a new solid phase. The nucleation occurs once the surface free energy barrier posed by the new solid-liquid interface is overcome by the free energy win from bulk phase growth.
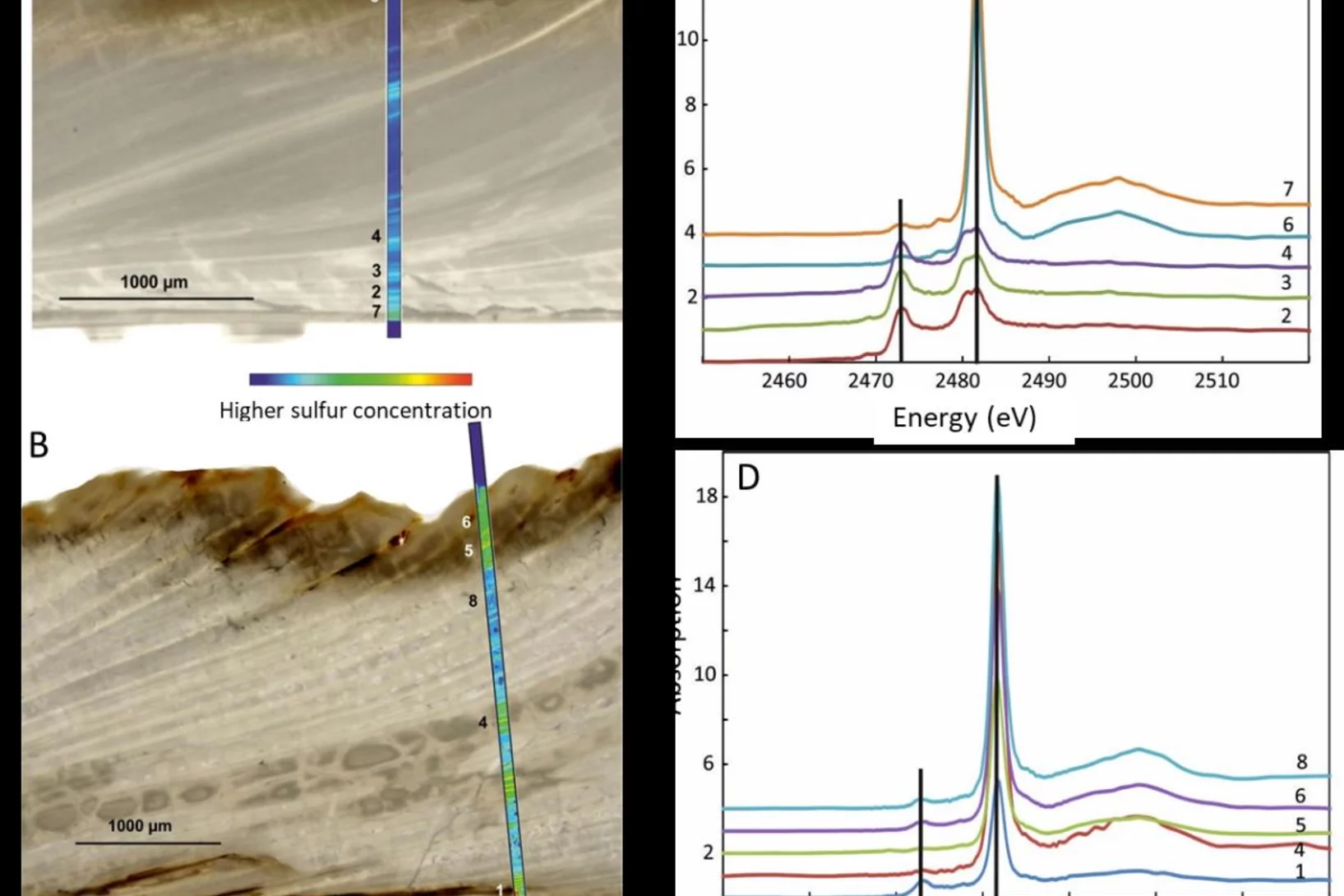
How is sulfur incorporated in biogenic carbonates and how is it affected by hydrothermal alteration?
Sulfate in biogenic carbonates is an important proxy for reconstructing the marine sulfur cycle. To investigate the exact location of carbonate associated sulfate (CAS) in biogenic carbonates and the effects of diagenetic alteration on sulfur in carbonates, shells of the marine bivalve Arctica islandica were artificially altered in modified seawater. Sulfur XANES analyses showed that CAS in A. islandica is indeed incorporated into the mineral part of the pristine shell, most likely as a hydrated or partly hydrated sulfate phase. The multi-analytical approach of XANES and µ-XRF analyses, sulfur isotope measurements, NanoSIMS analyses, and microstructural analysis on thin-sections of the shell samples further revealed that the different sulfur in a bivalve shell sensitively reacts to artificially induced hydrothermal alteration.
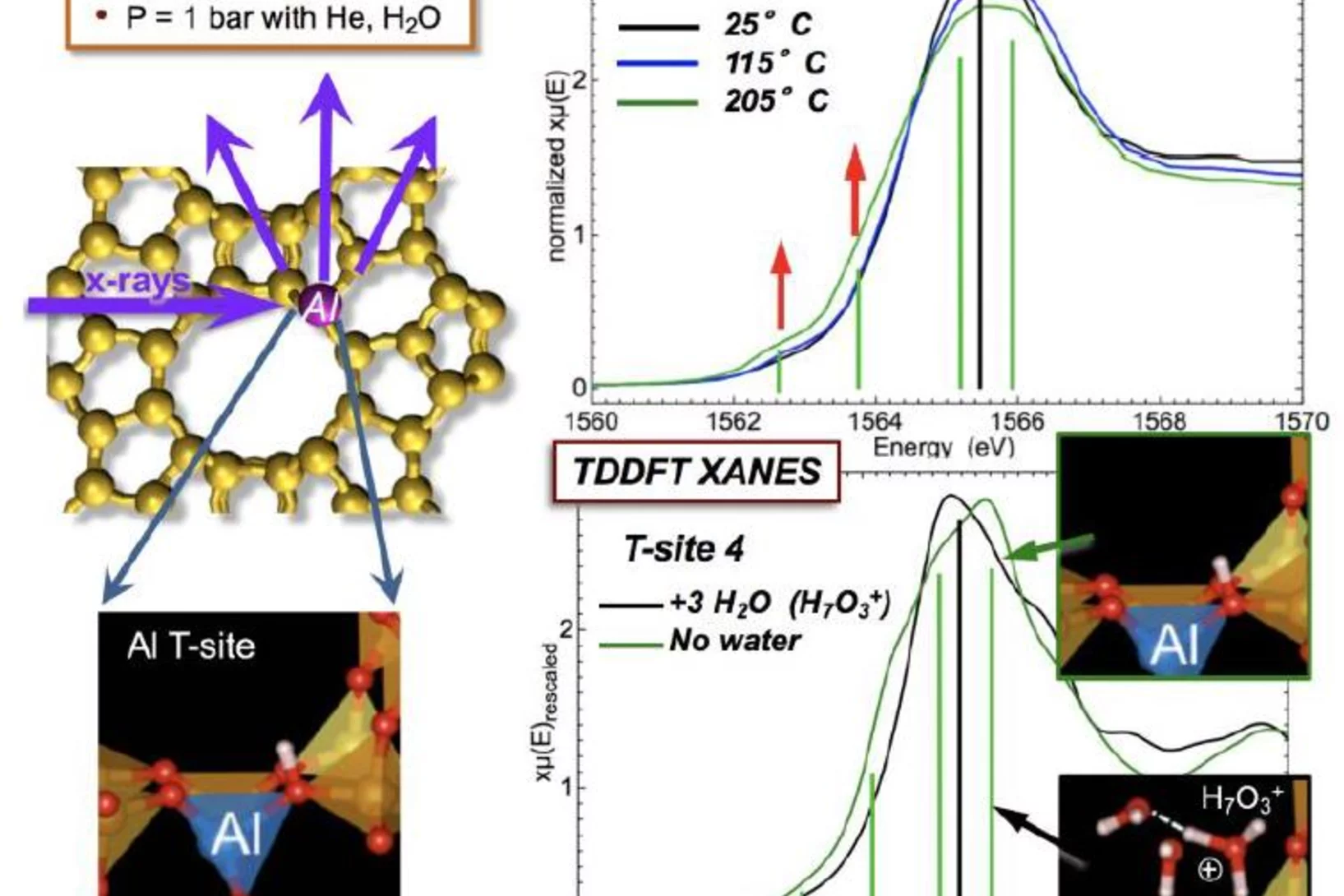
Tracking the Chemical Transformations at a Zeolite Brønsted Acid Site with Al K-edge XANES
Al T-sites are of crucial importance for the function of zeolite catalysts. These T-sites, which serve as Brønsted acid reaction centers, interact strongly with water. The location of these T-sites and their chemical state in the presence of water were elucidated using x-ray absorption spectroscopy (XAS) at the PHOENIX beamline at the Swiss Light Source of the PSI.
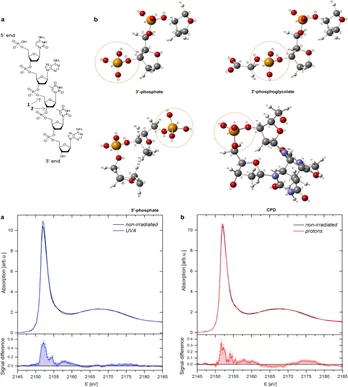
Biophysical effects of UV radiation on biological samples
The biological influence of radiation on living matter has been studied for years; however, several questions about the detailed mechanism of radiation damage formation remain largely unanswered. Among all biomolecules exposed to radiation, DNA plays an important role because any damage to its molecular structure can affect the whole cell and may lead to chromosomal rearrangements resulting in genomic instability or cell death.
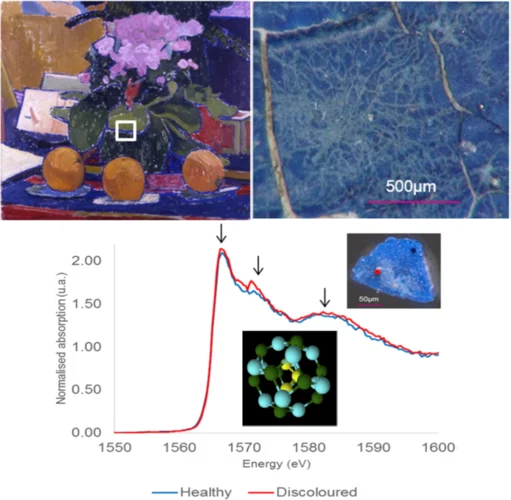
Aluminium X-ray absorption near-edge spectroscopy analysis of discoloured ultramarine blue in 20th century oil paintings
A specific case of synthetic ultramarine degradation was observed in three oil paintings from the early 20th century. Pigment particleswere found to have been discoloured, resulting in intricate patterns ofwhite lines, approximately 10 to 30 microns wide, criss-crossing the paint surface. Colour in ultramarine pigments comes from the encapsulated sulphur radical anions, chromophores, inside the cage framework built from SiO4 4 − and AlO4 5 −
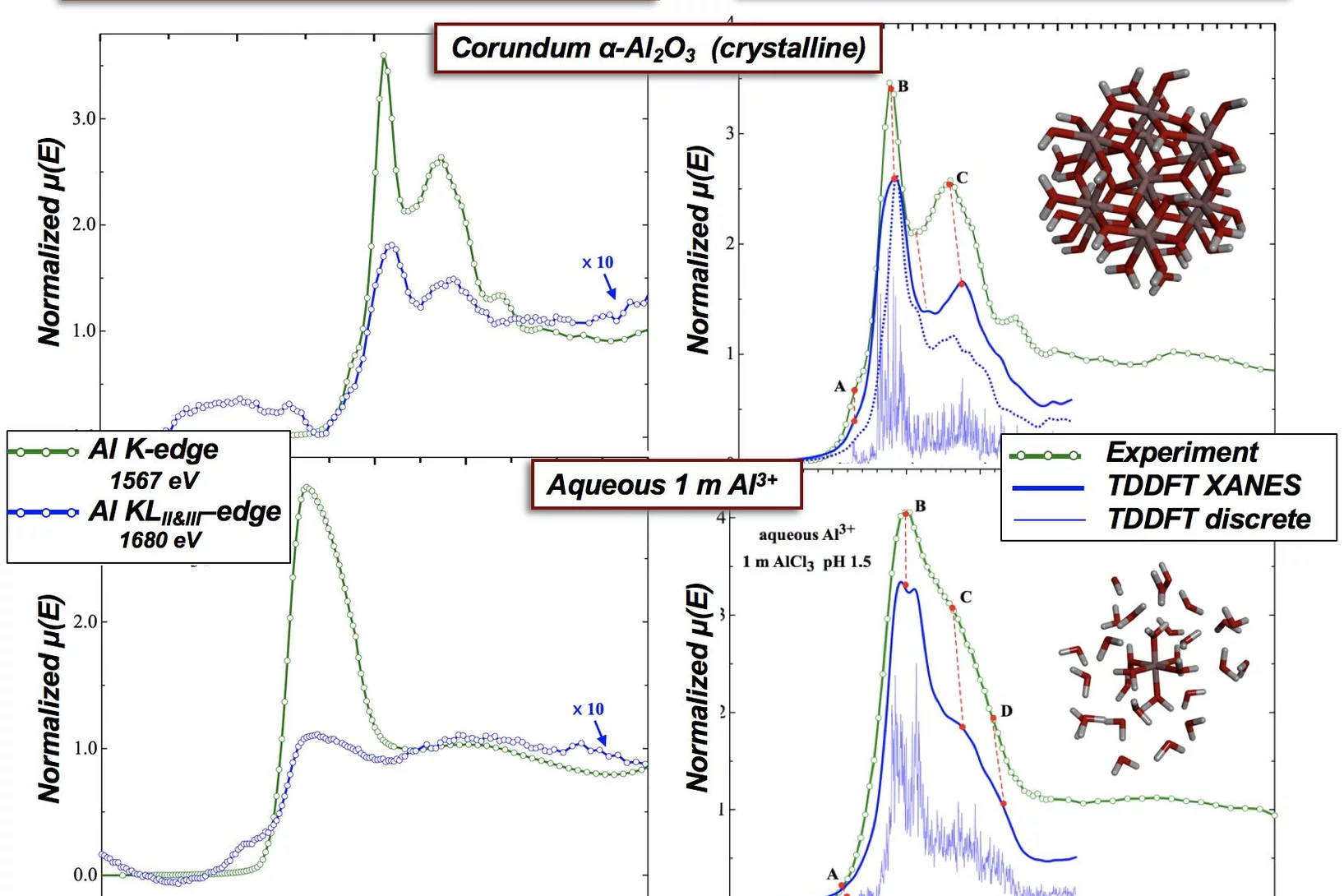
Single- (K) and Double-Electron Excitation (KLII&III) XANES Spectra of α-Alumina and Aqueous Al3+•(H2O)6
X-ray absorption spectroscopy (XAS) probes the local environment around an atom by study of the local photoelectron’s scattering. Multielectron excitations become more important at higher x-ray dose, which are used for examples in x-FEL experiments. Here we demonstrate that multielectron excitations, observed in the Al K-edges EXAFS spectra can be used to derive structural information.
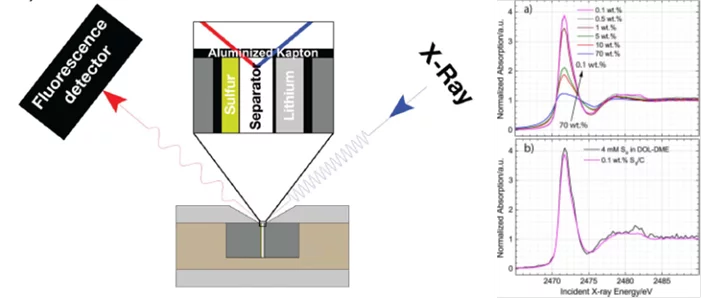
Operando Characterization of a LiS Battery
One of the technological barriers to electrification of transport is the insufficient storage capacity of the Li-ion batteries on which the current electric cars are based. The lithium-sulfur (Li-S) battery is an advanced technology whose successful commercialization can lead to significant gains in the storage capacity of batteries and promote wide-spread adoption of electric vehicles.
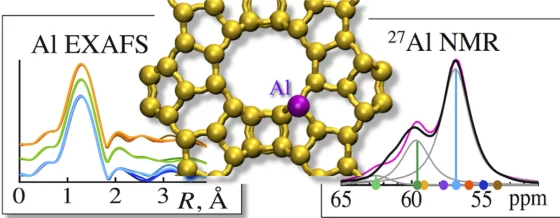
Quantitatively Probing the Al Distribution in Zeolites
The degree of substitution of Si4+ by Al3+ in the oxygen-terminated tetrahedra (Al T-sites) of zeolites determines the concentration of ion-exchange and Brønsted acid sites. Because the location of the tetrahedra and the associated subtle variations in bond angles influence the acid strength, quantitative information about Al T-sites in the framework is critical to rationalize catalytic properties and to design new catalysts.